Abstract
Background
Clinical manifestations of alcohol abuse on the cardiac muscle include defective contractility with the development of heart failure. Interestingly, low alcohol consumption has been associated with reduced risk of cardiovascular disease. Although several hypotheses have been postulated for alcoholic cardiomyopathy and for the low-dose beneficial cardiovascular effects, the precise mechanisms and mediators remain largely undefined. We hypothesize that modulation of oxidative stress by PI3K/Akt plays a key role in the cardiac functional outcome to acute alcohol exposure.
Methods
Thus, acutely exposed rat cardiac tissue and cardiocytes to low (LA: 5 mM), moderate (MA: 25 mM), and high (HA: 100 mM) alcohol were assessed for markers of oxidative stress in the presence and absence of PI3K/Akt activators (IGF-1 0.1 μM or constitutively active PI3K: Ad.BD110 transfection) or inhibitor (LY294002 1 μMor Akt-negative construct Ad.Akt(K179M) transfection).
Results
Acute LA reduced Akt, superoxide dismutase (SOD-3) and NFκB, ERK1, and p38 MAPK gene expression. Acute HA only increased that of SOD-3 and NFκB. These effects were generally inhibited by Ad.Akt(K179M) and enhanced with Ad.BD110 transfection. In parallel, LA reduced but HA enhanced Akt activity, which was reversed by IGF-1 and inhibited by Ad.Akt(K179M), respectively. Also, LA reduced caspase 3/7 activity and oxidative stress, while HA increased both. The former was blocked, while the latter effect was enhanced by Ad.Akt(K179M). The reverse was true with PI3K/Akt activation. This translated into reduced viability with HA, with no effect with LA. On the functional level, acute LA improved cardiac output and ejection fraction, mainly through increased stroke volume. This was accompanied with enhanced end-systolic pressure–volume relationship and preload recruitable stroke work. Opposite effect was recorded for HA. LA and HA in vivo functional effects were alleviated by LY and enhanced by IGF-1 treatment.
Conclusions
Acute LA and HA seem to oppositely affect cardiac function through modulation of oxidative stress where PI3K/Akt plays a pivotal role.
Keywords: Alcohol, PI3K, Akt, Oxidative Stress, Contractility, Cardiac
Heavy acute alcohol exposure has been shown to negatively affect the function of many organs in the body and alter oxidative stress responses in the cardiovascular system (El-Mas and Abdel-Rahman, 2014). In particular, binge drinking is a common social form of acute alcoholic intoxication that has been shown to exert detrimental cardiovascular effects (Tonelo et al., 2013; Waszkiewicz et al., 2013). Nonetheless, reports have also demonstrated beneficial effects of low alcohol (LA) exposure (Djousse et al., 2004; Providencia, 2006; Reims et al., 2004). It has been shown that acute high alcohol (HA) exposure increases cellular oxidative stress through alterations in the AMPK and the mTOR signaling activity (Kandadi et al., 2013; Zhang et al., 2010). However, the proper mechanism(s) through which low and high levels of acute alcohol alters the cardiac function is not well deciphered.
Clinically, acute heavy alcohol consumption depresses cardiac function and modifies local blood flow (Kandadi et al., 2013; Reims et al., 2004). There are many reports, which demonstrate that alcohol-induced cardiomyopathy is facilitated through the generation of oxidative stress (Kannan et al., 2004; Preedy et al., 1999). Other clinical parameters affected by acute and chronic alcohol intake include left ventricular malfunction, thinning of ventricular wall, and myocardial dysfunction (Fogle et al., 2011).
LA consumption has been shown to have beneficial effects on cardiovascular function and lower risk of heart disease. Adversely, chronic HA consumption has various detrimental effects leading to alcoholic cardiomyopathy (Krenz and Korthuis, 2012). Our laboratory and others have linked the Akt pathway as a mediator of alcohol-induced translational diminishment of anti-apoptotic signals (Chen et al., 2000; Haddad et al., 2008). Akt pathway has been heavily studied with its association with induced oxidative stress (Ma and Wang, 2012), which may be linked to cardiomyopathy. The phase II antioxidant enzymes are up-regulated defending against oxidative stress (Akhtar et al., 2012). Alcohol effects on the anti-apoptotic and cell survival pathways have yet to be elucidated.
The contractile cardiomyocytes are greatly affected by high doses of ethanol (EtOH) (100 to 200 mM) leading to apoptosis and later necrosis (>24 hours) (Altura et al., 1996; Doser et al., 2009; Guan et al., 2004; Kandadi et al., 2013). Apoptotic changes are mainly orchestrated through caspase 3 (Porter and Janicke, 1999). The anti-apoptotic pathway is up-regulated through PI3K/Akt via a series of phosphorylation steps when stress signals are perceived from external or internal stimuli. During acute HA exposure, there is a remarkable increase in protein phosphatase (PP2A), which leads to decreases in Akt (Chen et al., 2010;Ma et al., 2010). The sarcomere also becomes damaged interfering with its inotropic activity, which leads to cardiac muscle dysfunction (Chen et al., 2010). Thus, we hypothesize that Akt signaling pathway plays a pivotal role in mediating the detrimental versus the beneficial effects of HA versus LA exposure on the heart function, respectively.
MATERIALS AND METHODS
Conformity Statement
All the procedures conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH) publication No. 85-23, revised 1996; and approved by the IACUC of Howard University (Supporting Information).
RESULTS
Effects of Acute Alcohol on Gene Expression of the Apoptotic and Anti-Apoptotic Signaling Pathways
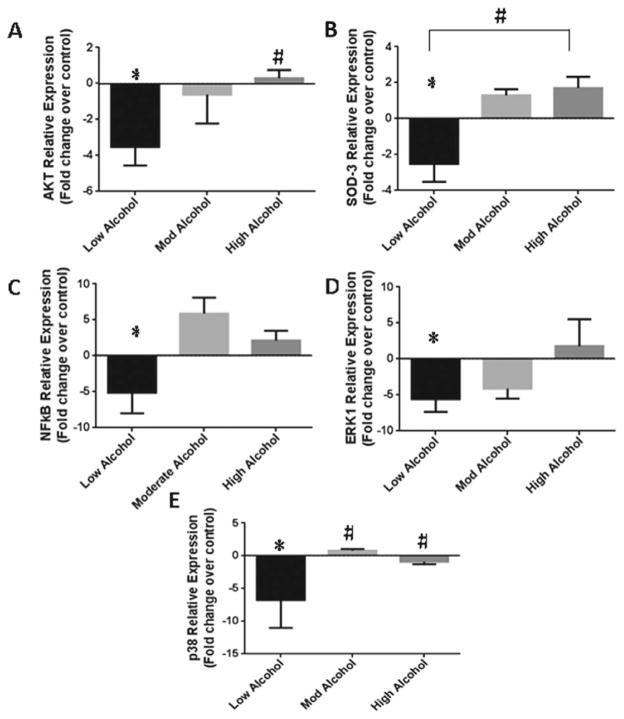
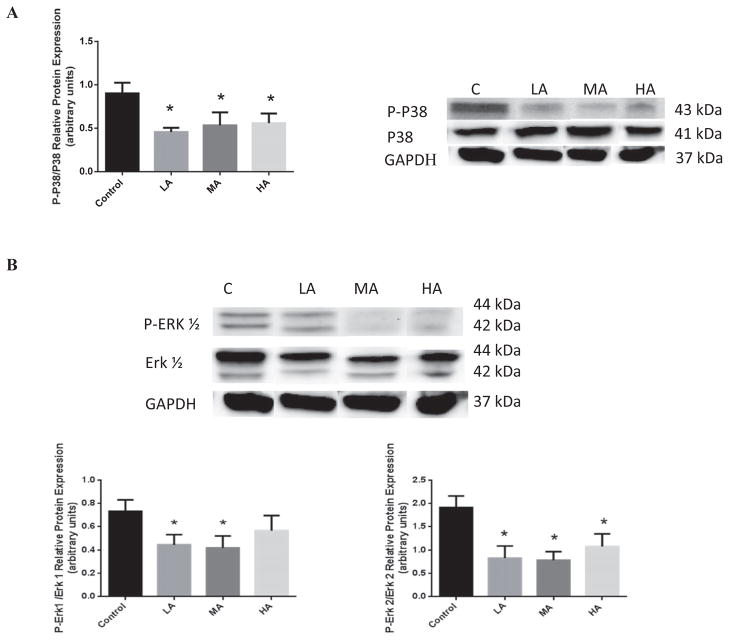
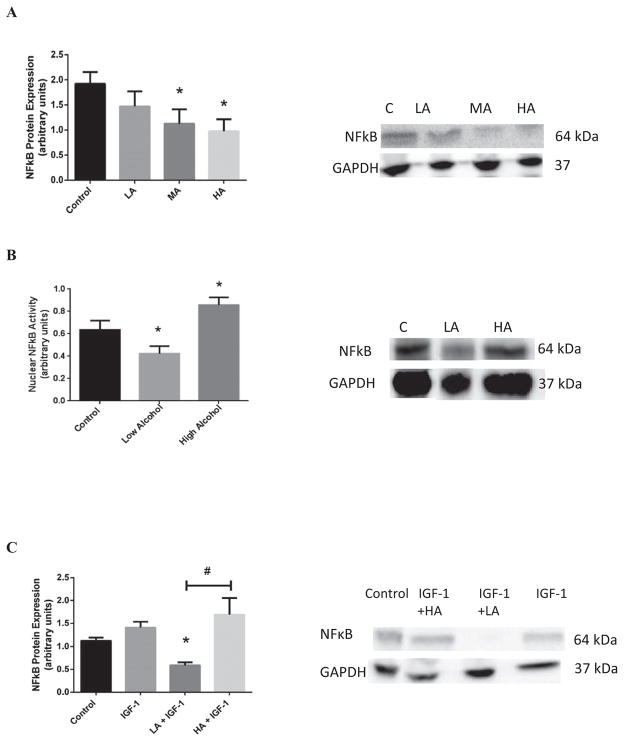
We first assessed the importance of the PI3K/Akt and the MAPK (ERK1/2 and p38) pathways in mediating the alcohol-induced acute effects on apoptotic signaling by exposing the hearts to different levels of alcohol. In Fig. 1A, we show that LA decreased Akt gene expression by 3.5 ± 1.0-fold (p < 0.001), whereas HA did not alter Akt expression as compared to control. As alcohol can affect the level of reactive oxygen species (ROS) antioxidant, we monitored the phase II antioxidant enzyme, superoxide dismutase (SOD-3) gene expression (Fig. 1B). The data show that high levels of alcohol result in 1.7 ± 0.6-fold increase in SOD-3 gene expression (p < 0.001), while LA greatly diminishes SOD-3 by 2.5 ± 1.0-fold (p < 0.002). NFκB, a universal transcription regulator, may act with the PI3K/Akt pathway to transduce oxidative stress signals. Therefore, we monitored NFκB gene expression (Fig. 1C) to show the alcohol effects on transcription rate. We found that LA diminishes the gene expression of NFκB by 5.1 ± 3.2-fold (p < 0.002), while moderate alcohol (MA) and HA have a trend to increase NFκB gene expression by 5.9 ± 2.5- and 2.1 ± 1.5- folds as compared to control samples, respectively.
Fig. 1.
Acute alcohol levels can affect the Akt/PI3K pathway. (A–E) Akt, SOD-3, NFkB, ERK1, and p38 relative gene expression with low, moderate, and high levels of alcohol treatment. Fold change in expression shown as compared to control samples (*) and low alcohol (#) with p-values < 0.05.
In parallel, we further investigated the modulation of MAPK by alcohol, such as the p38 MAPK and the ERK1/2, which are responsive to stress stimuli (Reinking et al., 2009; Zhao et al., 2010a). We observed that LA reduces ERK1 gene expression by 5.6 ± 1.8-fold (p < 0.01), whereas MA and HA had no effect (Fig. 1D). Similar effects of acute LA exposure were seen with p38 MAPK gene expression (Fig. 1E), which diminished by 6.7 ± 4.2-fold as compared to control samples (p < 0.05).
Modulation of the Alcoholic Effects on Apoptotic and Anti-Apoptotic Signaling by PI3K/Akt
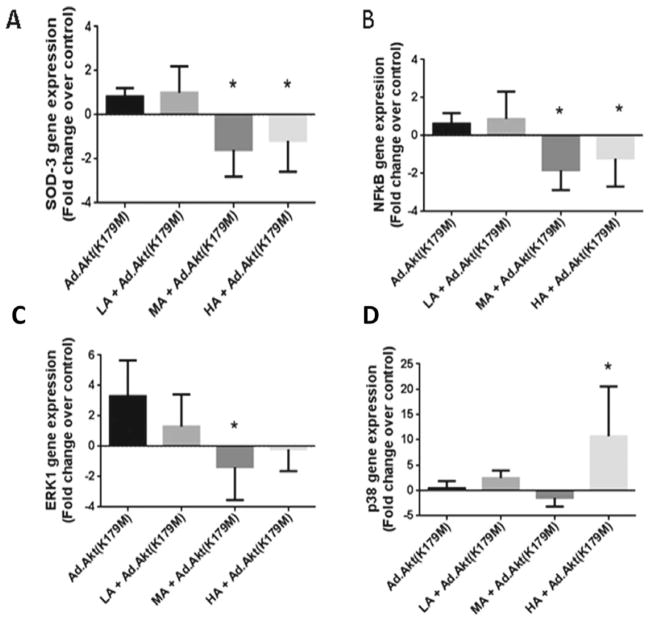
We then utilized adenovirus constructs to administer cassettes containing coding to overexpress or underexpress Akt levels inside cardiomyocytes. As depicted in Fig. 2A, transfection of the heart with dominant-negative Akt alleviated the LA-induced reduction in ROS with a SOD-3 gene expression level of 1.0 ± 0.6-fold of control; however, compared with acute LA only, this elevation in SOD-3 gene expression generated 1.5-fold change (p < 0.03). On the other hand, MA and HA exposures of Ad.Akt(K179M)-transfected hearts decreased SOD-3 gene expression to 1.6 ± 0.6-fold (p < 0.02) and 1.2 ± 0.7-fold (p < 0.03) of control, respectively. Similarly, as shown in Fig. 2B, knocking down Akt alleviated the negative effect of LA on NFκB gene expression, whereas MA and HA exposure reduced NFκB expression (1.8 ± 0.52-fold, p < 0.007; 1.2 ± 0.7- fold, p < 0.049, respectively). The parallel pathway, ERK1, demonstrated similar responses as SOD and NFκB expression in response to different alcohol levels. Thus, knocking down Akt alleviated the reduction in ERK1 expression induced by acute LA (0.4 ± 1.2-fold vs. control), whereas MA maintained that reduction (1.4 ± 1.0-fold; p < 0.04) (Fig. 2C). Furthermore, p38 MAPK reduction by acute LA exposure was prevented by Ad.Akt(K179M) transfection. Interestingly, p38 MAPK shows a dramatic increase (10.7 ± 5.7-fold; p < 0.03) with exposure to HA when Akt is underexpressed (Fig. 2D).
Fig. 2.
Adenovirus-mediated transfection with Akt knockout can modulate alcohol effects on the Akt/PI3k pathway. (A–D) SOD-3, NFkB, ERK1, and p38 relative gene expression with acute low, moderate, and high levels of alcohol treatment along with adenovirus Ad.Akt (K179M, Akt knockout). Fold change in expression shown as compared to control samples (*) with p-values < 0.05. All Ad:EGFP control samples were equivalent to control nontransfected samples.
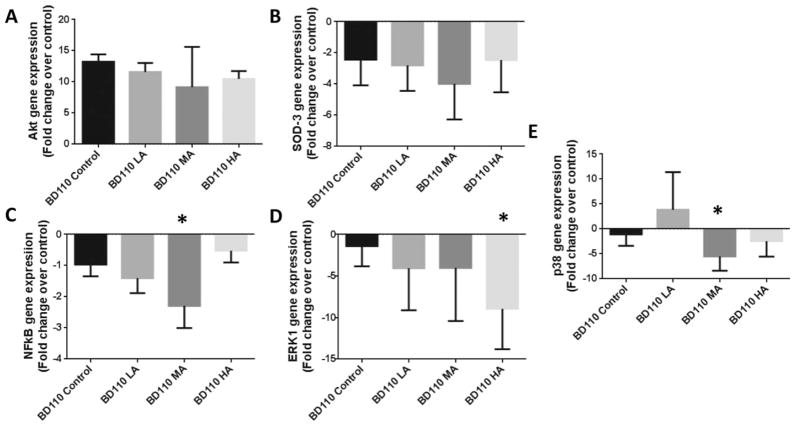
On the other hand, we examined the effects of the constitutive activation of PI3K in cardiomyocytes, with Ad.BD110 transfection, on the Akt, ERK1/2, and p38 MAPK gene expressions. As expected, we observed (Fig. 3A) elevated levels of Akt expression by 11.6 ± 0.9-, 9.2 ± 5.3-, and 9.9 ± 0.1-folds irrespective of alcohol treatment (all samples were not significantly different from the nonalcoholic Ad.BD110-transfected control, but significantly higher than nontransfected control [p < 0.05]). We then tested for SOD-3 expression with Ad.BD110-transfected samples. There was a general trend to decrease SOD-3 gene expression with PI3K activation irrespective of the alcohol level (Fig. 3B). Furthermore, Ad.BD110 transfection diminished the decrement in NFκB gene expression with LA compared with control (1.4 ± 0.2-fold) as shown in Fig. 3C. On the other hand, there was a general down-regulation of the ERK1 gene expression by 4.1 ± 1.8 and 4 ± 2.4-folds (p > 0.05 vs. control) and significant decline 8.9 ± 2.4-fold (p < 0.003 vs. control) in the presence of Ad.BD110 with LA, MA, and HA, respectively (Fig. 3D). In addition, we observed a decrease in p38 MAPK gene expression (5.5 ± 1.0-fold [p < 0.006] and 2.5 ± 1.0-fold) with MA and HA exposures when PI3K is up-regulated by co-treatment with Ad.BD110 (Fig. 3E).
Fig. 3.
Overexpression of Akt can alleviate the effects of various alcohol levels on key proteins in Akt/PI3K pathway. (A–E) Akt, SOD-3, NFkB, ERK1, and p38 relative gene expression with low, moderate, and high levels of alcohol along with Ad:BD110 treatment. Fold change in expression shown as compared to control samples (*) with p-values < 0.05. All Ad:EGFP control samples were equivalent to control nontransfected samples.
Effects of Alcohol on Protein Levels and Activities of Apoptotic and Anti-Apoptotic Signaling Pathways
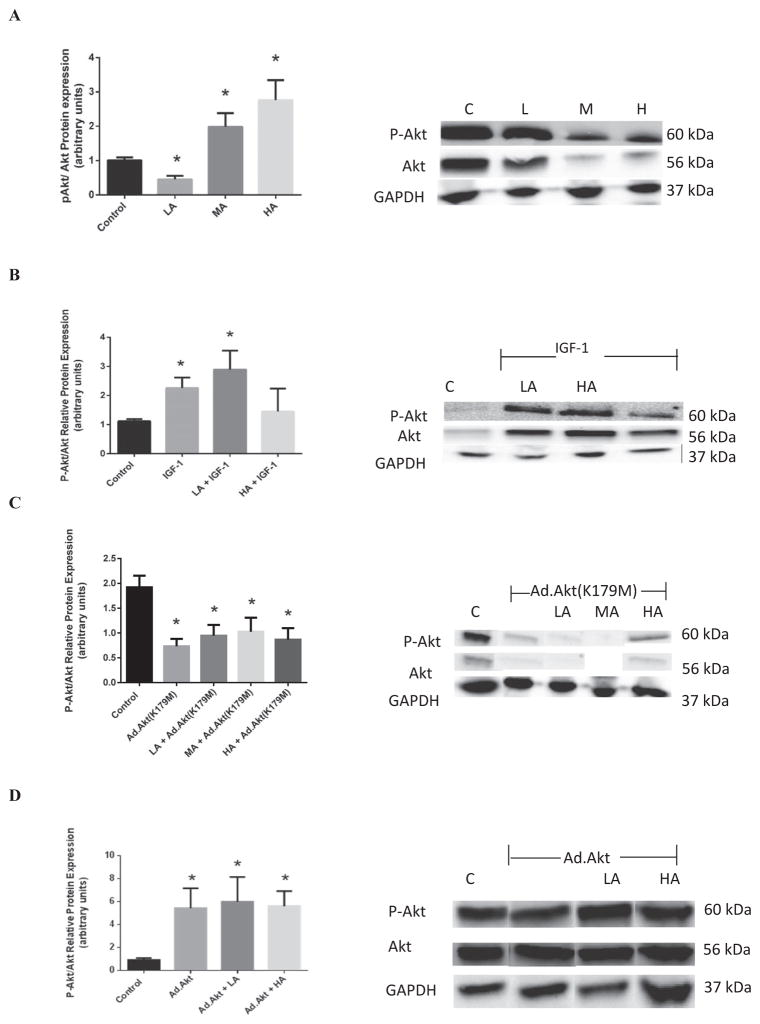
Protein expression and activities of apoptotic and anti-apoptotic signaling molecules were assessed to show the effects on transcription in the presence of the alcohol treatments (Fig. 4). As shown in Fig. 4A, Akt activity was increased by 97.3 ± 0.81% (p < 0.02) and 175 ± 0.87% (p < 0.004) with MA and HA, whereas LA reduced the Akt activity by 55.2 ± 7.47%(p < 0.008).
Fig. 4.
Effects of acute alcohol on the Akt activity in the heart depicted as the ratio of phosphorylated Akt over total Akt protein expressions. (A) The differential Akt activity in control (C), low alcohol (LA),moderate alcohol (MA), and high alcohol (HA)-exposed hearts, with representative gels (representative figure in left panel). (B) The effects of exogenous PI3K activator, IGF-1, on cardiac Akt activity in the presence of alcohol (representative gels in right panel). (C) The effects of transfection of heart tissue with dominant-negative Akt, Ad.Akt(K179M) on the Akt activity in the presence of alcohol (representative figure in left panel). (D) The effects of Ad.Akt on cardiac Akt activity in the presence of alcohol (representative gels in right panel). n = 3; *p < 0.05 compared with control.
Figure 4B shows that IGF-1, known PI3K/Akt agonist, increased Akt activity in the nonalcoholic heart by 101.6 ± 0.3% (p < 0.02). Furthermore, when hearts were acutely exposed to LA, IGF-1 significantly increased Akt activity by 157.7 ± 0.3% (p < 0.02). Thus, IGF-1 alleviated the negative effects of LA on Akt activity in the heart. On the other hand, knocking down of Akt with Ad.Akt(K179M) inhibited the effects of alcohol on the cardiac Akt activity (Fig. 4C). Thus, there was no significant difference between LA, MA, or HA in the presence of Ad.Akt(K179M) versus nonalcoholic control.
P38 and ERK1/2 are parallel MAPK pathways that respond to several stress stimuli. As shown in Fig. 5A, LA, MA, and HA significantly reduced p38 activity by 97.6 ± 0.33% (p < 0.04), 69.0 ± 0.54% (p < 0.05), and 61.3 ± 0.05% (p < 0.03), respectively. In addition, we also observed that LA and MA exposure reduced ERK1 activity by 64.3 ± 0.47% (p < 0.04) and 74.8 ± 0.47% (p < 0.03), respectively, as compared to control samples. HA did not exert an effect (Fig. 5B). Similar effects of acute LA and MA exposure were seen with ERK2 activity (Fig. 5B), which diminished by 131.7 ± 0.35%(p < 0.02) and 145.0 ± 0.30% (p < 0.01), respectively. Interestingly, HA also reduced ERK2 activity by 78.2 ± 0.46%(p < 0.04).
Fig. 5.
Effects of acute alcohol exposure on MAPK (P38 and ERK1/2) protein expression in the heart. (A) The P38 protein expression in control C, low alcohol (LA), moderate alcohol (MA), and high alcohol (HA)-exposed hearts, with representative gels (in right panel). (B) The ERK1/2 protein expression in C, LA, MA, and HA-exposed hearts with representative gels (in right panel) (n = 3; *p < 0.05 compared with control).
In addition, we investigated the relevance of NFκB in the cardiac response to different levels of alcohol. As shown in Fig. 6A, MA and HA exposure significantly reduced NFκB activity by 41.4 ± 0.4% (p < 0.05) and 49.2 ± 0.3% (p < 0.02), respectively. Accordingly, Fig. 6B shows that LA reduced NFκB nuclear translocation (33.4 ± 5.3%; p < 0.05), while HA enhanced it (35.0 ± 6.1%; p < 0.05). The negative effects of HA on the NFκB activity were significantly reversed by co-treatment with IGF-1 (Fig. 6C). Actually, NFκB nuclear activities were normalized with IGF-1 treatment.
Fig. 6.
Effects of acute alcohol exposure on the NFκB protein expression in the heart. (A) NFκB protein expression in control (C), low alcohol (LA), moderate alcohol (MA), and high alcohol (HA)-exposed hearts, with representative gels (in right panel). (B) The nuclear NFkB protein expression in C, LA, and HA-exposed hearts with representative gels (in right panel). (C) The effects of exogenous PI3K activator, IGF-1, on cardiac NFκB protein expression in the presence of alcohol (representative gels in right panel) (n = 3; *p < 0.05 compared with control; #p < 0.05 compared with LA).
Effects of Alcohol on Cellular Apoptosis
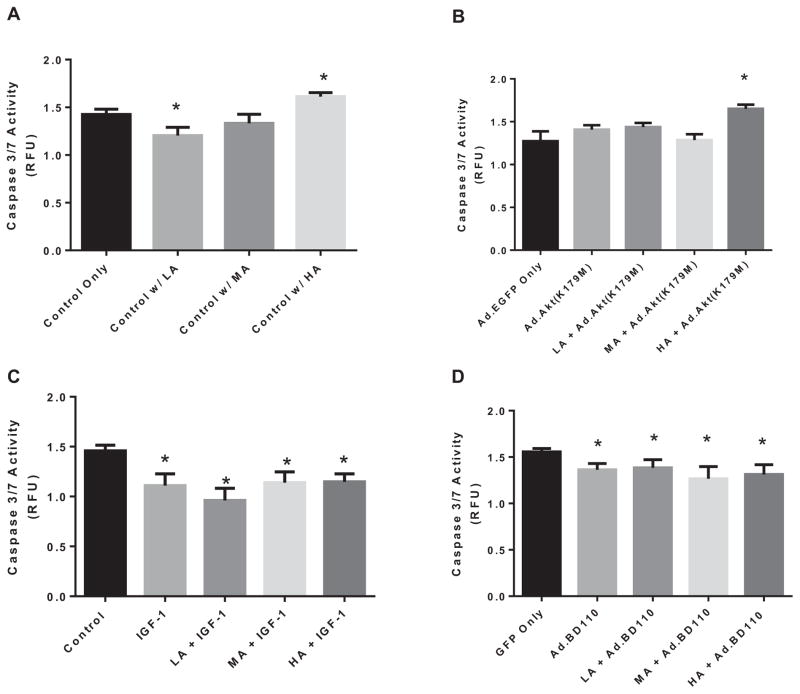
We investigated the effects of alcohol on the initiation of cellular death by evaluating caspase 3/7 activity, which are known to play a critical role in the initiation of death in cardiomyocytes (Yue et al., 1998). Thus, we monitored the level of caspase 3/7 activity on cardiomyocytes that were treated with varying levels of alcohol alone, or in combination with Ad.EGFP, Ad.Akt(K179M), Ad.BD110, or IGF-1. As shown in Fig. 7A, acute LA exposure reduced caspase 3/7 activity by 15.4 ± 0.4% (p < 0.04) as compared to control. MA exposure did not have a significant effect on caspase 3/7 activity. However, acute exposure of the cardiomyocytes to HA significantly increased caspase 3/7 activity by 13.0 ± 0.37% (p < 0.04).
Fig. 7.
Caspase 3/7 activity from isolated cardiomyocytes that were treated with varying levels of alcohol (low [LA], moderate [MA], or high [HA]) alone (A) or in combination with Ad.Akt(K179M) (B), IGF-1 (C), or Ad.BD110 (D). n = 3, *p < 0.05 compared with control.
On the other hand, knocking down the Akt gene (Fig. 7B) with Ad.Akt(K179M) blocked the reduction in caspase 3/7 activity induced by LA; however, it intensified the increase in caspase 3/7 activity induced by HA (22.8 ± 0.34%; p < 0.02, as compared to control).
Conversely, we investigated the effects of PI3K/Akt pathway activation by IGF-1 on the regulation of caspase 3/7 activity by varying levels of alcohol (Fig. 7C). IGF-1 alone decreased caspase 3/7 activity in nonalcoholic cardiomyocytes by 23.8 ± 0.3% (p < 0.04). Addition of IGF-1 prevented the negative alterations in caspase 3/7 activity previously seen with alcohol exposures. Thus, in the presence of IGF-1, there was a reduction in caspase 3/7 activity by 34.0 ± 0.27% (p < 0.03), 21.7 ± 0.39% (p < 0.04), and 21.2 ± 0.3% (p < 0.02) with LA, MA, and HA, respectively.
Similarly, the constitutive activation of PI3K via Ad.BD110 transfection resulted in a significant decrease in apoptotic induction as assessed by caspase 3/7 activity. Figure 7D shows that cardiocytes co-treated with LA and Ad.BD110 exhibited a reduction in caspase 3/7 activity by 15.0 ± 0.3% (p < 0.02). Co-treatment of MA and HA with Ad.BD110 significantly reduced caspase 3/7 activity by 19.8 ± 0.45% (p < 0.05) and 16.9 ± 0.4% (p < 0.04), respectively.
Effects of Alcohol on Cellular Viability
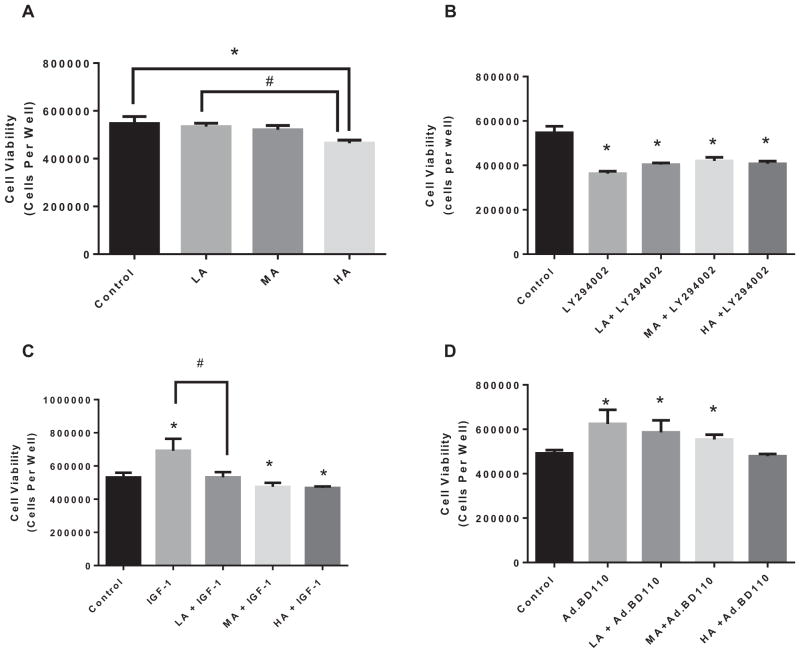
To assess the effects of apoptosis on cell survival, we have investigated the effects of alcohol on cellular viability of isolated cardiomyocytes that were treated with varying levels of alcohol alone, or in combination with Ad.EGFP, Ad.BD110, IGF-1, or LY294002. There was no significant effect on cell viability (Fig. 8A) with LA. HA exposure significantly decreased cell viability by 14.9 ± 0.3% (p < 0.03) compared with control. Inhibiting PI3K, via LY294002 (Fig. 8B) resulted in an overall decrease in cellular viability. Accordingly, cardiomyocytes survival decreased by 26.1 ± 0.2% (p < 0.0002), 23.1 ± 0.2% (p < 0.002), and 25.5 ± 0.2% (p < 0.0002), with LA, MA, and HA, respectively, in the presence of PI3K inhibition.
Fig. 8.
Cell viability from isolated cardiomyocytes that were treated with varying levels of alcohol (low [LA],moderate [MA], or high [HA]) alone (A) or in combination with LY294002 (B), IGF-1 (C) or Ad.BD110 (D). n = 3; *p < 0.05 compared with control. #p < 0.05 as compared to IGF-1.
As expected, IGF-1 by itself caused a significant increase in cell survival by 30.1 ± 0.4% (p < 0.004), indicating that IGF-1 enhances cardiomyocytes survival (Fig. 8C). This increase in IGF-1-induced cell viability was reduced (23.0 ± 0.5%; p < 0.05) by LA co-treatment. Thus, there was no significant difference with LA in the presence of IGF-1 compared with control. In addition, IGF-1 co-treatment with MA and HA exposure seems to significantly reduce cardiomyocytes survival by 10.6 ± 0.6% (p < 0.03) and 11.8 ± 0.4% (p < 0.04), respectively. Therefore, the negative effects seen with MA and HA were not removed by IGF-1.
Cardiomyocytes transfected with Ad.BD110, showed an increased Akt expression with LA exposure associated with a significant increase in cell viability by 16.1 ± 0.5% (p < 0.05), compared with control (Fig. 8D). Similar results were found with MA exposure and Ad.BD110 transfection (11.3 ± 0.4%; p < 0.05). There were no significant differences in cell survival with HA + Ad.BD110; that is, PI3K activation seems to counteract the effects of HA on cardiomyocytes viability.
Effects of Alcohol on Oxidative Stress
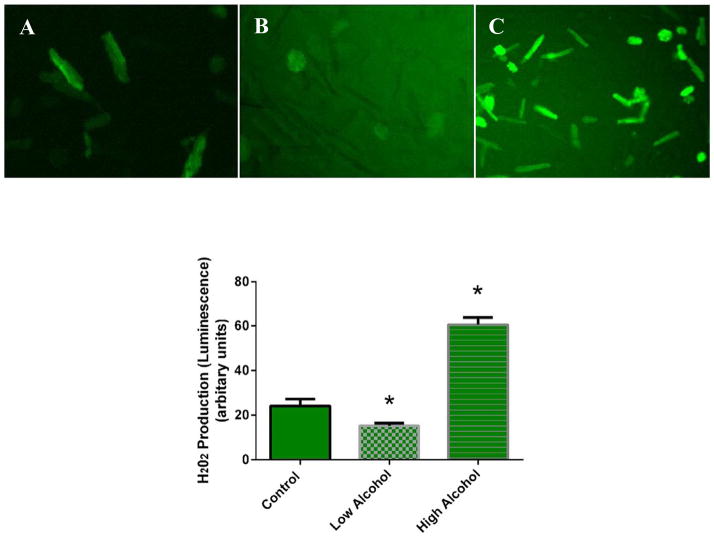
We evaluated oxidative stress levels on isolated cells that were treated with varying concentrations of alcohol. As shown in Fig. 9, H2O2 production was significantly decreased by LA 36.8 ± 0.4% (p < 0.04). In the presence of HA, H2O2 production was significantly increased by 150.4 ± 0.2% (p < 0.002). These results indicate that LA can decrease while HA can increase oxidative stress in cardiomyocytes.
Fig. 9.
Qualitative images of cardiomyocytes exhibiting reactive oxygen species (ROS) production. Fluorescent images were captured at the maximal signal with each alcohol exposure: (A) control, (B) low alcohol, and (C) high alcohol. The peak of fluorescence occurred within 1 to 2 minutes after exposure to different amounts of alcohol. (D) Quantitative analysis of the maximal signal with each alcohol exposure. Bars represent mean values ± SEM (n = 6; *p < 0.05 compared with control).
Effects of Alcohol on Cardiac Function In Vivo
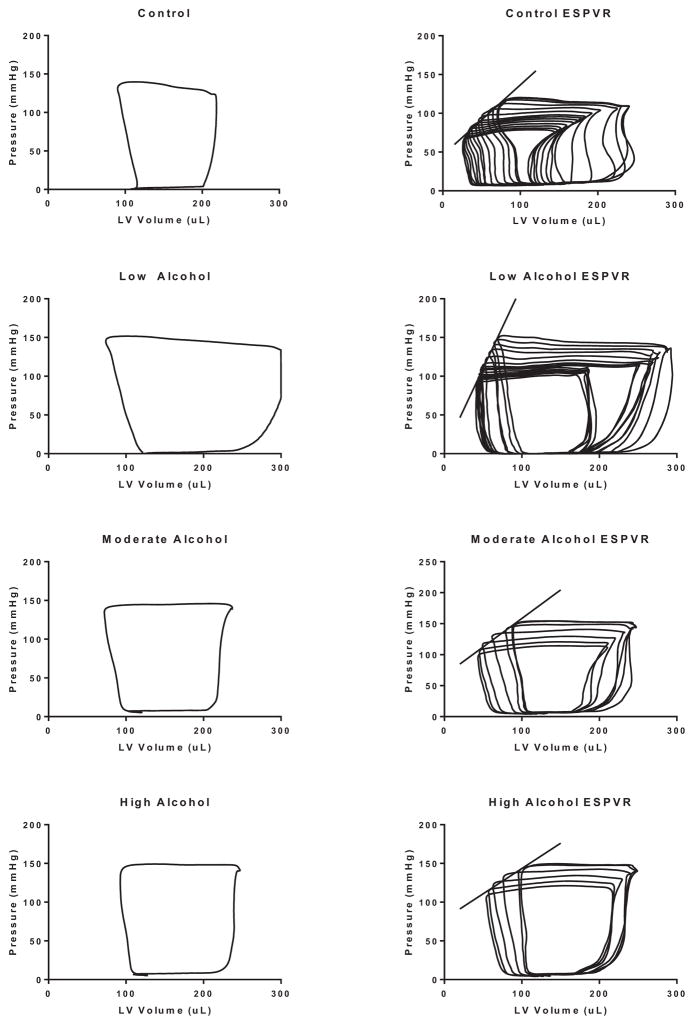
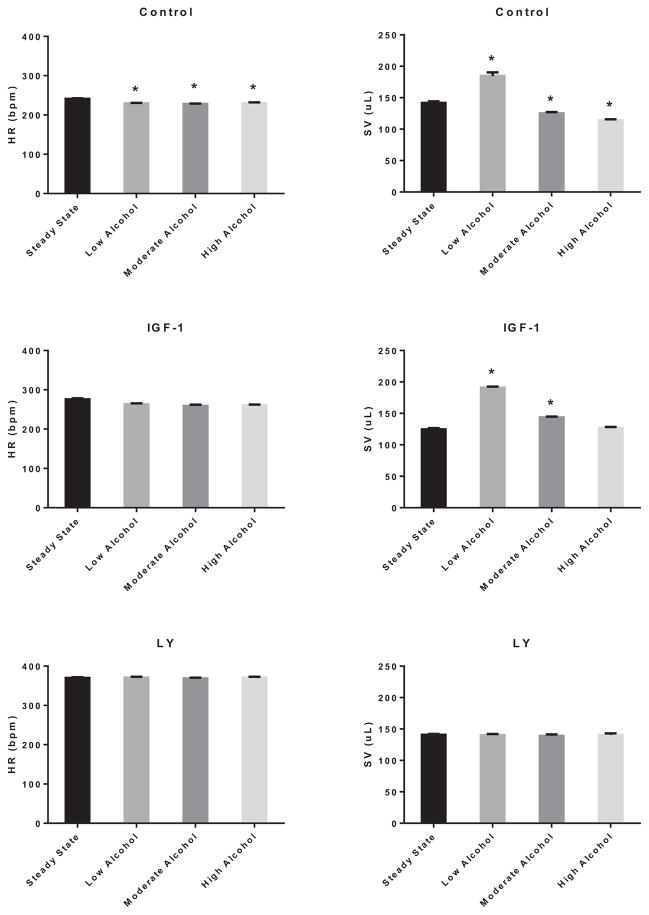
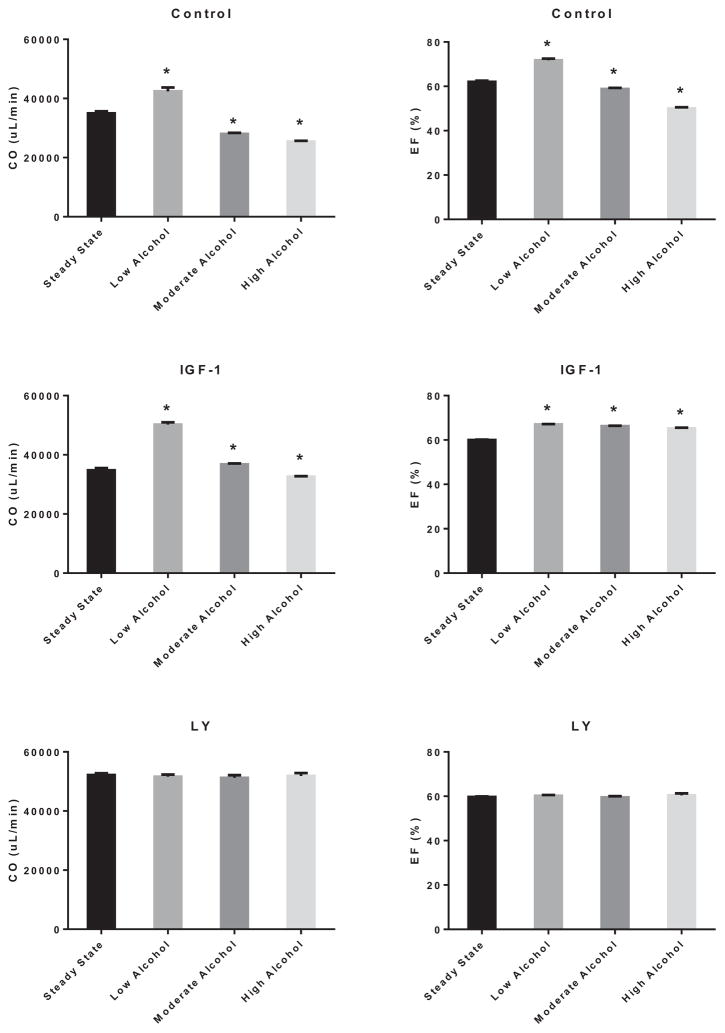
Figure 10 (left panels) shows representative LV pressure–volume (PV) loops of control, LA, MA, and HA treated hearts, while the right panels depict the generation of regressive PV loops via preload reduction. As shown in Fig. 11, acute treatment with EtOH slightly reduced the heart rate irrespective of its concentration by 4.9 ± 0.2% (LA), 5.4 ± 0.1% (MA), and 4.4 ± 0.2% (HA) (p < 0.05). Interestingly, the stroke volume (SV) was improved by LA (30.3 ± 0.1%; p < 0.01) but reduced by MA (11.7 ± 0.2%; p < 0.05) and HA (19.7 ± 0.1%; p < 0.05). Activation of PI3K did not alter the negative effect of acute alcohol on the heart rate. However, IGF-1 alleviated the detrimental effects of MA and HA and enhanced that of LA on the SV (LA: 52.9 ± 0.1%, p < 0.05; MA: 15.0 ± 0.1, p < 0.05). Inhibition of PI3K by LY294002 alleviated the alcohol effects on these parameters. As shown in Fig. 12, this translated into an increase in the cardiac output (CO) by 21.2 ± 0.2% (p < 0.05) and ejection fraction (EF) by 15.8 ± 0.1% (p < 0.05) with LA, but reduction in CO (19.8 ± 0.1%; p < 0.05 and 27.3 ± 0.1%; p < 0.05) and EF (5.1 ± 0.2; p < 0.05 and 19.3 ± 0.1%; p < 0.05) with MA and HA, respectively. In the presence of IGF-1, the CO was further enhanced by LA (44.5 ± 0.1; p < 0.01), slightly improved by MA (5.8 ± 0.4; p < 0.05), but still reduced by HA (6.2 ± 0.4%; p < 0.05). Nonetheless, there was general improvement of the EF parameter by IGF-1 with all acute alcohol exposures (LA: 11.7 ± 0.1%, MA: 10.3 ± 0.1, and HA: 8.7 ± 0.1%; p < 0.06). However, inhibition of PI3K alleviated the effects of acute alcohol on these parameters.
Fig. 10.
Effects of acute low alcohol (LA), moderate alcohol (MA), or high alcohol (HA) exposures on pressure–volume (PV) relationships of the left ventricle of adult male rats. Left panel: representative steady-state PV loop for control heart (first row), LA-exposed heart (second row),MA-exposed heart (third row), and HA-exposed heart (fourth row). Right panel: representative regression of PV loops following occlusion of the vena cava where the slope of the regression line depicts the end-systolic PV relationship (ESPVR) for each condition, respectively.
Fig. 11.
Effects of alcohol on heart rate (HR) and stroke volume (SV). Heart rate (left panel) and SV (right panel) modulation by low alcohol (LA), moderate alcohol (MA), and high alcohol (HA) alone (upper row), in combination with IGF-1 (middle row), or with LY294002 (lower row). n = 4; *p < 0.05 compared with control.
Fig. 12.
Effects of alcohol on cardiac output (CO) and ejection fraction (EF). Cardiac output (left panel) and EF (right panel) modulation by low alcohol (LA), moderate alcohol (MA), and high alcohol (HA) alone (upper row), in combination with IGF-1 (middle row), or with LY294002 (lower row). n = 4; *p < 0.05 compared with control.
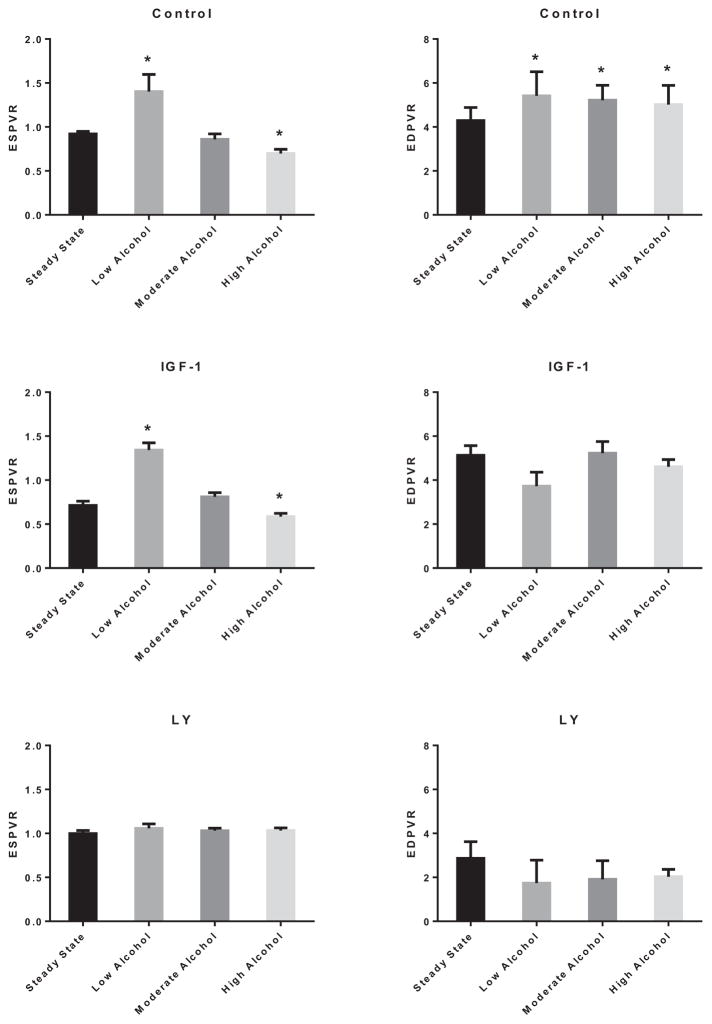
Analysis of the end-systolic PV relationship (ESPVR) (Fig. 13) shows enhancement of the LV contractility with acute LA (134.0 ± 0.3%; p < 0.01); however, there was reduction in ESPVR with acute HA exposure (24.1 ± 0.4%; p < 0.05). Furthermore, the end-diastolic PVR (EDPVR) was significantly increased with acute alcohol exposure irrespective of its concentration to a similar extent (3.68 ± 0.60 mm Hg/μl for control vs. 5.42 ± 1.09 mm Hg/μl for LA, 5.22 ± 0.68 mm Hg/μl for MA, and 5.02 ± 0.87 mm Hg/μl for HA; p < 0.05). Interestingly, inhibition of PI3K blocked the alcohol-induced changes to ESPVR and EDPVR.
Fig. 13.
Effects of alcohol on left ventricular end-systolic pressure–volume relationship (ESPVR) and end-diastolic PVR (EDPVR). ESPVR (left panel) and EDPVR (right panel) modulation by low alcohol (LA), moderate alcohol (MA), and high alcohol (HA) alone (upper row), in combination with IGF-1 (middle row), or with LY294002 (lower row). n = 4; *p < 0.05 compared with control.
DISCUSSION
Through this work, we have demonstrated that (i) Akt acts as a mediator in cardiac function in the presence of alcohol as well as (ii) low levels of acute alcohol can be beneficial through reduction in oxidative stress and cell death; (iii) MAPK ERK1/2 acts as an auxiliary pathway when Akt pathway is obliterated; however, it is unable to completely compensate for the Akt role in cardiac function; (iv) activation of Akt can overcome most of the effects that acute MA and HA levels have on oxidative stress and survival key regulators in cardiac function; (v) acute HA has detrimental effects on oxidative stress levels, cell viability, and caspase activity; (vi) acute LA improves cardiac function in vivo through enhance contractility and SV, while acute HA has the opposite effect; (vii) PI3K/Akt plays a pivotal role in mediating the alcohol effects on cardiac function in vivo.
Accordingly, we have shown that different levels of alcohol exposure in the heart have significantly distinct effects on the cell survival signaling associated with the PI3K/Akt pathway. To that effect, we have determined that acute LA decreases Akt activity. Hence, LA may diminish the necessity for the PI3K/Akt pathway to be activated which also correlates with the Akt gene expression. Nevertheless, HA activation of Akt may inhibit AMPK leading to contractile dysfunction (Cai et al., 2008). Similarly, Ren (2007) has demonstrated that acute EtOH treatment in a murine diet also induces a decrement in Akt expression while up-regulating the phase II antioxidant protein, FOXO-3 in cardiomyocytes. We also have observed that Akt down-regulation reveals a compensatory enhancement of the ERK1/2 protein levels. As this proliferative pathway is parallel to PI3K/Akt, it may act in an auxiliary capacity. This is in agreement with our previous work indicating a similar cross-talk between Akt and ERK activity; in particular, inhibition of the PI3K/Akt pathway induced an up-regulation in ERK1/2 activity (Zhao et al., 2010a). Yet, the reduction in Akt gene expression and activity with LA does not seem to acutely affect cell viability, but did reduce oxidative stress. This may be due to the very limited natural ability of cardiomyocytes to proliferate, especially in a short period of time. In the same line, LA also decreases apoptotic events which is further diminished with IGF-1 co-treatment and overactivation of PI3K. Therefore, LA seems to exert a protective/preventative effect on cellular survival and function. On the other hand, acute HA enhanced the Akt activity as seemingly an auto-regulatory mechanism against the detrimental effects of HA. In support of this, adding IGF-1 further increases Akt expression and activity enough to effectively alleviate the damaging effects of HA on cardiomyocytes survival and apoptotic events. This finding is consistent with other groups that were able to also identify IGF-1 as a protective molecule against cardiac apoptosis (Fazio et al., 1996; Frustaci et al., 1992). This evidence in combination with our findings suggests that IGF-1 can protect against alcohol- induced cardiomyopathy, even in the presence of higher doses of alcohol. Conversely, 1 group was able to show a protective measure of growth hormone in reducing apoptosis in neonatal rat cardiomyocytes; however, they were unable to identify IGF-1 as mediating this effect (Gonzalez-Juanatey et al., 2004). This may be due to the fact that the experiments were performed with neonatal cardiomyocytes which differ in signaling from adult cardiomyocytes.
It is well known that IGF-1/PI3K/Akt work together to induce multiple biological effects in the heart including cell survival and hypertrophy (Matsui and Davidoff, 2007). Accordingly, our data have demonstrated that the effects of alcohol on oxidative stress and cardiomyocyte survival can be alleviated by transfection with dominant-negative Akt. Conversely, similar observations were made with adenoviral transfection using Ad.BD110, a constitutively active form of PI3K, showing the alleviation of HA detrimental effects on cardiomyocytes survival and occurrence of apoptosis. Thus, it seems that the effects of alcohol (low and high) are mainly mediated via PI3K. We have shown that the biomarker for cellular apoptosis, caspase 3/7 activity, was significantly decreased in the presence of LA-treated cardiomyocytes, as well as those overexpressing Akt (Ad.BD110) and treated with IGF-1. These results suggest that LA can reduce apoptotic activity via the PI3K/Akt pathway. Our findings are consistent with those of other research, suggesting that low doses of EtOH activate the PI3K/Akt pathway in H9c2 cultured cardiomyocytes (Ma et al., 2010; Zhao et al., 2010b). In contrast, our data also show that caspase activity is increased in the presence of cardiomyocytes treated with HA, as well as cardiomyocytes transfected with Ad.Akt (K179M). These finding suggest that HA increases caspase activity via the PI3K/Akt pathway. This may also correspond with the decline in cardiomyocyte viability seen with HA and with PI3K inhibition. These results suggest that when the PI3K/Akt pathway is inhibited, LA is unable to exert a protective/preventative effect.
EtOH activation of NFκB has been linked to the modulation of downstream genes effects (Kojima et al., 2012) as well as pro-inflammatory cytokines during up-regulation of oxidative stress (Pedruzzi et al., 2012). Another group showed that physical stress to the heart could induce NFκB in cardiomyocytes (Leychenko et al. 2011). Here, we demonstrated that NFκB gene expression was decreased in the presence of LA in heart tissue along with reduced nuclear translocation. This reduction in expression was attenuated by PI3K activation and inhibited by dominant-negative Akt. Thus, NFκB may mediate the downstream Akt-dependent alcohol effects. Interestingly, constitutive activation of PI3K induced a consistent reduction in NFκB gene expression. Research shows that PI3K/Akt works as a negative feedback loop preventing excessive inflammatory responses that require NFκB to activate downstream immune-related factors such as iNOS and COX-2 (Yang et al., 2013). Importantly, HA did not alter NFκB gene expression, but enhanced its protein nuclear translocation from the cytoplasm. This demonstrates a positive regulatory effect of NFκB during HA exposure. Furthermore, we also showed that the negative effect of HA on NFκB activity was reversed by co-treatment of IGF-1. This is consistent with our data indicating the transduction of the Akt-dependent alcoholic effects in the heart may be partially mediated via NFκB.
The PI3K/Akt pathway has been implicated in modulating oxidative stress levels (Ma and Wang, 2012). In this article, we showed that the antioxidant protein, SOD-3, had decreased gene expression in the presence of LA-treated heart tissue, which correlated with the observed reduction in the levels of ROS production in the cytoplasm of LA-treated cardiomyocytes. Conversely, in the presence of HA ROS production significantly increased in cardiomyocytes along with enhanced SOD-3 gene expression. Akt knockout experiments showed that the effects of LA and HA on SOD-3 could be mediated through the PI3K/Akt pathway. This is further consolidated as constitutive activation of PI3K averted the anticipated ROS response to alcohol. Other groups have shown that Akt inhibition may elicit an induction of SOD expression (Akca et al., 2013; Lijnen et al., 2010). However, we have shown a reduction in SOD-3 expression with MA and HA in Akt knockout hearts. This may indicate the strong dependence of SOD-3 response on Akt activation in these cardiomyocytes. As a correlate, it has been demonstrated that SOD activity can regulate the expression of the stress signaling molecule, p38 (Gutierrez-Uzquiza et al., 2012). In our experiments, p38 was down-regulated by LA, which was alleviated by Akt knockout and reversed by PI3K activation. This is in agreement with recent work showing that Akt negatively regulates p38 (Yang et al., 2013). Furthermore, other groups have implicated p38 in the onset of heart failure (Sharov et al., 2003). In support to our findings, it has been recently shown that acute alcohol detrimental inotropic effects on the heart are associated with increased ROS and enhanced Akt and ERK activities, which were reversed by ERK inhibition in vivo (El-Mas and Abdel-Rahman, 2014). Thus, it seems that alcohol-induced oxidative stress on the heart elicits protective survival pathways against its detrimental effects. In parallel, other positive inotrope second messengers such as PKC and consequently AMPK have been reported to be inhibited by heavy EtOH (Liangpunsakul et al., 2008). The interplay between different pathways converges on a reduction in contractile function of the myocardial cells.
MAPK plays a heavy role in relaying stress signals in cardiomyocytes. ERK1/2 and p38 are among the MAPKs that modulate gene expression (Walker et al., 2013). Our data showed that ERK1 mRNA levels are diminished with LA treatment. However, the obliteration of Akt reverses this effect. Conversely, when Akt is overactivated, ERK1 is drastically diminished with all levels of alcohol. We and others have recently shown that there is a compensatory activation of ERK1/2 when PI3K/Akt pathway is down-regulated (Ham and Mahoney, 2013; Zhao et al., 2010a). Others have also demonstrated significant cross-talk between PI3K/Akt and its parallel ERK pathway (Dai et al., 2009). Akt also regulates p38/ERK in an inhibitory fashion (Yang et al., 2013). However, as PI3K independently can activate MAPKs, there may be auto-regulation occurring when Akt is overexpressed.
In vivo assessment of cardiac function reveals an enhancement of the inotropic activity of the heart with LA, while a detrimental one exists with HA. Alcohol-induced changes in the EF reflected changes in the SV, which was increased by LA and decreased by HA. Aistrup and colleagues (2006) have previously reported a decline in the excitation–contraction coupling associated with acute HA. In addition, it was recently shown in dogs (Fenelon et al., 2007) that acute EtOH depresses LV function. Another group (Campbell et al., 2000) has shown a reduction in the chronotropic and inotropic activity of the rat heart with acute exposure to EtOH. These findings can be translated into a weaker force of contraction and therefore reduced SV. A general increase in EDPVR indicates an impaired diastolic function (increased stiffness) with acute alcohol exposure, which seems to be positively counter balanced by enhanced contractility with LA leading to greater EF. However, this increased stiffness with HA was not concomitant with enhancement of the ESPVR, which yielded a decrement in EF. Accordingly, it was reported that acute MA intake does not affect myocardial contractility in human (Kupari et al., 1983). It is important to mention the inhalatory/volatile anesthetic, isoflurane has been reported to decrease ventricular contractility and heart rate per se (Blaudszun and Morel, 2012). This can exacerbate the HA reported negative effects but underestimate the positive effects of LA on contractility. However, the same dose of isoflurane was used for the control nonalcohol treated as well as for the LA and HA group, comparing our alcoholic PV loop data (hemodynamics) of alcohol-treated groups to the nonalcoholic control ones could mitigate the anesthetic effect. On the other hand, in agreement with our molecular data, we have also shown that the PI3K/Akt pathway seems to play an important regulatory role in mediating the inotropic effects of alcohol. This is substantiated with the alleviation of the alcohol inotropic effects by PI3K inhibition as well as the alleviation of HA detrimental inotropic effect by PI3K activation. In support of that, a recent publication (Zeng et al., 2012) showed that PI3K activation may mediate the acute EtOH effect. It is important to differentiate between the reported beneficial effects of chronic low doses of alcohol consumption and the acute effects found in this study. Long-term exposure to alcohol has an inherent cumulative and “adaptational” response which is not part of the acute effects. This is especially true for infrastructural changes related to the extracellular environment and remodeling. The acute responses may reflect in part a transient cardiac response in the absence of recurrent exposure. In this study, the acute effect was restricted to 30 minutes to mimic the alcohol loading time in social drinkers, peaking at 30 minutes from a single alcohol dose (Childs et al., 2011), and to limit the HA cellular damage as longer experiments were not consistently feasible. This is also in agreement with studies on acute HA reporting their findings within similar time frame (Childs et al., 2011; El-Mas and Abdel-Rahman, 2014). Finally, as we have shown in this study the importance of oxidative stress in inducing myocardial dysfunction, other synchronic mechanisms of EtOH could be as important. It has been shown that chronic EtOH exposure reduces the myosin ATPase activity in cardiac muscle (Reddy and Beesley, 1990), induces transition from alpha- to beta-myosin heavy chain (Meehan et al., 1999), deregulates nuclear translocation of RACK-1 scaffolding protein (Vagts et al., 2003), and alters cardiac matrix metalloproteinase function leading to enhanced collagen deposit (El Hajj et al., 2014). This study reveals an appreciation for the pivotal role of the PI3K/Akt in mediating the beneficial as well as the detrimental effects of LA and HA on the cardiac function, respectively, through the management of the oxidative stress. Future studies differentiating the effects of alcohol in pathophysiological conditions will assist in producing specific targets for heart failure.
Supplementary Material
Acknowledgments
The authors thank Ms. Maia Warner for helping in PV loop data processing and graphs.
FUNDING
This work was supported in part by grants 1 R15 AA019816-01A1 and 2G12 RR003048 RCMI, Division of Research Infrastructure to GEH.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Data S1. Material and methods.
References
- Aistrup GL, Kelly JE, Piano MR, Wasserstrom JA. Biphasic changes in cardiac excitation–contraction coupling early in chronic alcohol exposure. Am J Physiol Heart Circ Physiol. 2006;291:H1047–H1057. doi: 10.1152/ajpheart.00214.2006. [DOI] [PubMed] [Google Scholar]
- Akca H, Demiray A, Aslan M, Acikbas I, Tokgun O. Tumour suppressor PTEN enhanced enzyme activity of GPx, SOD and catalase by suppression of PI3K/AKT pathway in non-small cell lung cancer cell lines. J Enzyme Inhib Med Chem. 2013;28:539–544. doi: 10.3109/14756366.2011.654114. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Yousif MH, Chandrasekhar B, Benter IF. Activation of EGFR/ERBB2 via pathways involving ERK1/2, P38 MAPK, AKT and FOXO enhances recovery of diabetic hearts from ischemia-reperfusion injury. PLoS One. 2012;7:e39066. doi: 10.1371/journal.pone.0039066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Zou LY, Altura BT, Jelicks L, Wittenberg BA, Gupta RK. Beneficial vs. detrimental actions of ethanol on heart and coronary vascular muscle: roles of Mg2+ and Ca2+ Alcohol. 1996;13:499–513. doi: 10.1016/0741-8329(96)00044-4. [DOI] [PubMed] [Google Scholar]
- Blaudszun G, Morel DR. Superiority of desflurane over sevoflurane and isoflurane in the presence of pressure-overload right ventricle hypertrophy in rats. Anesthesiology. 2012;117:1051–1061. doi: 10.1097/ALN.0b013e31826cb20b. [DOI] [PubMed] [Google Scholar]
- Cai Y, Wang Q, Ling Z, Pipeleers D, McDermott P, Pende M, Heimberg H, Van de Casteele M. Akt activation protects pancreatic beta cells from AMPK-mediated death through stimulation of mTOR. Biochem Pharmacol. 2008;75:1981–1993. doi: 10.1016/j.bcp.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Campbell PH, Barker LA, McDonough KH. The effect of acute ethanol exposure on the chronotropic and inotropic function of the rat right atrium. J Pharm Pharmacol. 2000;52:1001–1010. doi: 10.1211/0022357001774723. [DOI] [PubMed] [Google Scholar]
- Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DB, Wang L, Wang PH. Insulin-like growth factor I retards apoptotic signaling induced by ethanol in cardiomyocytes. Life Sci. 2000;67:1683–1693. doi: 10.1016/s0024-3205(00)00759-1. [DOI] [PubMed] [Google Scholar]
- Childs E, O’Connor S, de Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcohol Clin Exp Res. 2011;35:1794–1803. doi: 10.1111/j.1530-0277.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol. 2009;34:1749–1757. doi: 10.3892/ijo_00000306. [DOI] [PubMed] [Google Scholar]
- Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D’Agostino RB, Wolf PA, Ellison RC. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–713. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38:448–456. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab. 2014;306:E740–747. doi: 10.1152/ajpendo.00465.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S, Biondi B, Sabatini D, Cuocolo A, Tommaselli AP, Lombardi G, Sacca L. Long-term growth hormone deficiency as a cause of cardiomyopathy and its reversibility with specific replacement therapy. J Clin Endocrinol Metab. 1996;81:887–890. doi: 10.1210/jcem.81.3.8772545. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Balbao CE, Fernandes R, Arfelli E, Landim P, Ayres O, Paola AA. Characterization of the acute cardiac electrophysiologic effects of ethanol in dogs. Alcohol Clin Exp Res. 2007;31:1574–1580. doi: 10.1111/j.1530-0277.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- Fogle RL, Hollenbeak CS, Stanley BA, Vary TC, Kimball SR, Lynch CJ. Functional proteomic analysis reveals sex-dependent differences in structural and energy-producing myocardial proteins in rat model of alcoholic cardiomyopathy. Physiol Genomics. 2011;43:346–356. doi: 10.1152/physiolgenomics.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Gentiloni N, Corsello SM, Caldarulo M, Russo MA. Reversible dilated cardiomyopathy due to growth hormone deficiency. Chest. 1992;102:326–327. doi: 10.1378/chest.102.1.326. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juanatey JR, Pineiro R, Iglesias MJ, Gualillo O, Kelly PA, Dieguez C, Lago F. GH prevents apoptosis in cardiomyocytes cultured in vitro through a calcineurin-dependent mechanism. J Endocrinol. 2004;180:325–335. doi: 10.1677/joe.0.1800325. [DOI] [PubMed] [Google Scholar]
- Guan Z, Lui CY, Morkin E, Bahl JJ. Oxidative stress and apoptosis in cardiomyocyte induced by high-dose alcohol. J Cardiovasc Pharmacol. 2004;44:696–702. doi: 10.1097/00005344-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Uzquiza A, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. p38alpha mediates cell survival in response to oxidative stress via induction of antioxidant genes: effect on the p70S6K pathway. J Biol Chem. 2012;287:2632–2642. doi: 10.1074/jbc.M111.323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GE, Saunders L, Carles M, Crosby SD, del MF, Macgillivray TE, Semigran MJ, Dec GW, Hajjar RJ, Doye AA, Glass R, ElM, Gwathmey JK. Fingerprint profile of alcohol-associated heart failure in human hearts. Alcohol Clin Exp Res. 2008;32:814–821. doi: 10.1111/j.1530-0277.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- Ham YM, Mahoney SJ. Compensation of the AKT signaling by ERK signaling in transgenic mice hearts overexpressing TRIM72. Exp Cell Res. 2013;319:1451–1462. doi: 10.1016/j.yexcr.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Kandadi MR, Hu N, Ren J. ULK1 plays a critical role in AMPK-mediated myocardial autophagy and contractile dysfunction following acute alcohol challenge. Curr Pharm Des. 2013;19:4874–4887. doi: 10.2174/1381612811319270010. [DOI] [PubMed] [Google Scholar]
- Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med (Maywood) 2004;229:553–559. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- Kojima S, Kuo TF, Tatsukawa H. Regulation of transglutaminase-mediated hepatic cell death in alcoholic steatohepatitis and non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2012;27(Suppl 2):52–57. doi: 10.1111/j.1440-1746.2011.07009.x. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. JMol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari M, Heikkila J, Ylikahri R. Acute effects of alcohol on left ventricular dynamics during isometric exercise in normal subjects. Clin Cardiol. 1983;6:103–108. doi: 10.1002/clc.4960060302. [DOI] [PubMed] [Google Scholar]
- Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 2011;6(12):e29055. doi: 10.1371/journal.pone.0029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S, Wou SE, Zeng Y, Ross RA, Jayaram HN, Crabb DW. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1173–G1181. doi: 10.1152/ajpgi.90349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen PJ, van Pelt JF, Fagard RH. Downregulation of manganese superoxide dismutase by angiotensin II in cardiac fibroblasts of rats: association with oxidative stress in myocardium. Am J Hypertens. 2010;23:1128–1135. doi: 10.1038/ajh.2010.128. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang H. PI3K/Akt/FoxO: a novel participant in signal transduction in bone cells under mechanical stimulation. Cell Biol Int. 2012;36:923–926. doi: 10.1042/CBI20120078. [DOI] [PubMed] [Google Scholar]
- Ma H, Yu L, Byra EA, Hu N, Kitagawa K, Nakayama KI, Kawamoto T, Ren J. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol. 2010;49:322–329. doi: 10.1016/j.yjmcc.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Davidoff AJ. Assessment of PI-3 kinase and Akt in ischemic heart diseases in diabetes. Methods Mol Med. 2007;139:329–338. doi: 10.1007/978-1-59745-571-8_22. [DOI] [PubMed] [Google Scholar]
- Meehan J, Piano MR, Solaro RJ, Kennedy JM. Heavy long-term ethanol consumption induces an alpha- to beta-myosin heavy chain isoform transition in rat. Basic Res Cardiol. 1999;94:481–488. doi: 10.1007/s003950050164. [DOI] [PubMed] [Google Scholar]
- Pedruzzi LM, Stockler-Pinto MB, Leite M, Jr, Mafra D. Nrf2-keap1 system versus NF-kappaB: the good and the evil in chronic kidney disease? Biochimie. 2012;94:2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Patel VB, Reilly ME, Richardson PJ, Falkous G, Mantle D. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999;4:e58–e66. doi: 10.2741/A480. [DOI] [PubMed] [Google Scholar]
- Providencia R. Cardiovascular protection from alcoholic drinks: scientific basis of the French Paradox. Rev Port Cardiol. 2006;25:1043–1058. [PubMed] [Google Scholar]
- Reddy YS, Beesley RC. Effects of acute and chronic ethanol on cardiac contractile protein ATPase activity of Syrian hamsters. Biochem Med Metab Biol. 1990;44:259–265. doi: 10.1016/0885-4505(90)90070-h. [DOI] [PubMed] [Google Scholar]
- Reims HM, Kjeldsen SE, Brady WE, Dahlof B, Devereux RB, Julius S, Beevers G, De FU, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Alcohol consumption and cardiovascular risk in hypertensives with left ventricular hypertrophy: the LIFE study. J Hum Hypertens. 2004;18:381–389. doi: 10.1038/sj.jhh.1001731. [DOI] [PubMed] [Google Scholar]
- Reinking BE, Wedemeyer EW, Weiss RM, Segar JL, Scholz TD. Cardiomyopathy in offspring of diabetic rats is associated with activation of the MAPK and apoptotic pathways. Cardiovasc Diabetol. 2009;8:43. doi: 10.1186/1475-2840-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. doi: 10.1002/9780470511848.ch5. [DOI] [PubMed] [Google Scholar]
- Sharov VG, Todor A, Suzuki G, Morita H, Tanhehco EJ, Sabbah HN. Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1. Eur J Heart Fail. 2003;5:121–129. doi: 10.1016/s1388-9842(02)00254-4. [DOI] [PubMed] [Google Scholar]
- Tonelo D, Providencia R, Goncalves L. Holiday heart syndrome revisited after 34 years. Arq Bras Cardiol. 2013;101:183–189. doi: 10.5935/abc.20130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagts AJ, He DY, Yaka R, Ron D. Cellular adaptation to chronic ethanol results in altered compartmentalization and function of the scaffolding protein RACK1. Alcohol Clin Exp Res. 2003;27:1599–1605. doi: 10.1097/01.ALC.0000089957.63597.A4. [DOI] [PubMed] [Google Scholar]
- Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, Haddad GE. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013;37:1253–1260. doi: 10.1111/acer.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszkiewicz N, Szulc A, Zwierz K. Binge drinking-induced subtle myocardial injury. Alcohol Clin Exp Res. 2013;37:1261–1263. doi: 10.1111/acer.12208. [DOI] [PubMed] [Google Scholar]
- Yang P, Han Y, Gui L, Sun J, Chen YL, Song R, Guo JZ, Xie YN, Lu D, Sun L. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-kappaB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochem Pharmacol. 2013;85:1124–1133. doi: 10.1016/j.bcp.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Yue TL, Wang C, Romanic AM, Kikly K, Keller P, DeWolf WE, Jr, Hart TK, Thomas HC, Storer B, Gu JL, Wang X, Feuerstein GZ. Staurosporine- induced apoptosis in cardiomyocytes: a potential role of caspase-3. JMol Cell Cardiol. 1998;30:495–507. doi: 10.1006/jmcc.1997.0614. [DOI] [PubMed] [Google Scholar]
- Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH, Zhu ZP, Xie KQ. PI3K/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology. 2012;296:56–66. doi: 10.1016/j.tox.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, Ren J. Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 2010;49:1238–1253. doi: 10.1016/j.freeradbiomed.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Alvin Z, Laurence G, Li C, Haddad GE. Cross-talk between MAPKs and PI-3K pathways alters the functional density of I(K) channels in hypertrophied hearts. Ethn Dis. 2010a;20(1 Suppl 1):S1–S24. [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Chu WF, Wu L, Li J, Liu QM, Lu YJ, Qiao GF, Wang ZG, Zhang ZR, Yang BF. PAF exerts a direct apoptotic effect on the rat H9c2 cardiomyocytes in Ca2+-dependent manner. Int J Cardiol. 2010b;143:86–93. doi: 10.1016/j.ijcard.2009.01.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.