Figure 2.
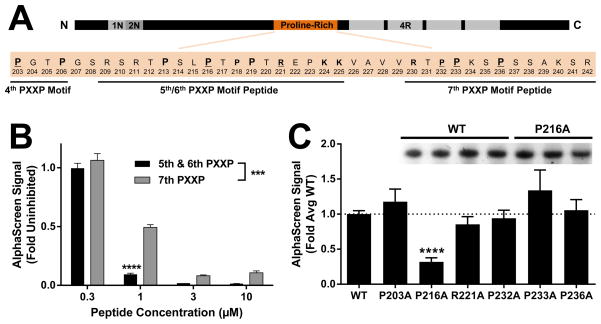
Fyn SH3 preferentially binds the 5th/6th PXXP motifs in Tau. (A) A portion of the proline-rich domain of Tau containing the 4th–7th PXXPs is shown. Residues potentially involved in polyproline helix binding are in bold and residues that were mutated for alanine scanning in panel C are underlined. Residues present in peptides spanning the 5th/6th or 7th PXXP motifs used in panel B are indicated. Primary prolines in the PXXP motifs are indicated by larger size. (B) Dose-response with Tat-peptides revealed significantly more potent inhibition by a peptide spanning the 5th/6th PXXPs than a peptide spanning the 7th PXXP. *** indicates p < 0.001 peptide effect by two-way ANOVA, and **** indicates p < 0.0001 by Sidak’s post hoc (n = 4 wells per group) vs. alternate peptide. Error bars indicate SEM. (C) An alanine scan revealed significant attenuation of the Tau–Fyn SH3 interaction by mutation of the proline shared by the 5th/6th PXXP motifs (P216A), but not by mutation of several residues in and around the 7th PXXP motif. ANOVA, p < 0.0001; **** indicates p < 0.0001 by Dunnett’s post hoc vs. WT control (n = 3–18 independent protein preparations per group). Error bars indicate SEM. (C, Inset) Representative protein concentration gel showing no difference between the concentration of WT and P216A protein preparations.