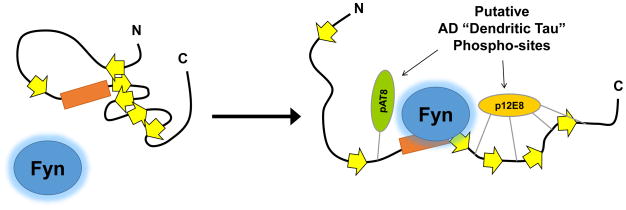
Figure 4.
Model for how disruption of Tau tertiary structure may facilitate Tau–Fyn SH3 interactions. Location of β-strands are approximate based on a published map of Tau secondary structure elements36. Under normal conditions, interactions between β-strands may help maintain Tau in a conformation that reduces access to the proline-rich domain. Phosphorylation may disrupt interactions between β-strands (right), opening the paperclip conformation of Tau and facilitating Fyn binding to the proline-rich domain.