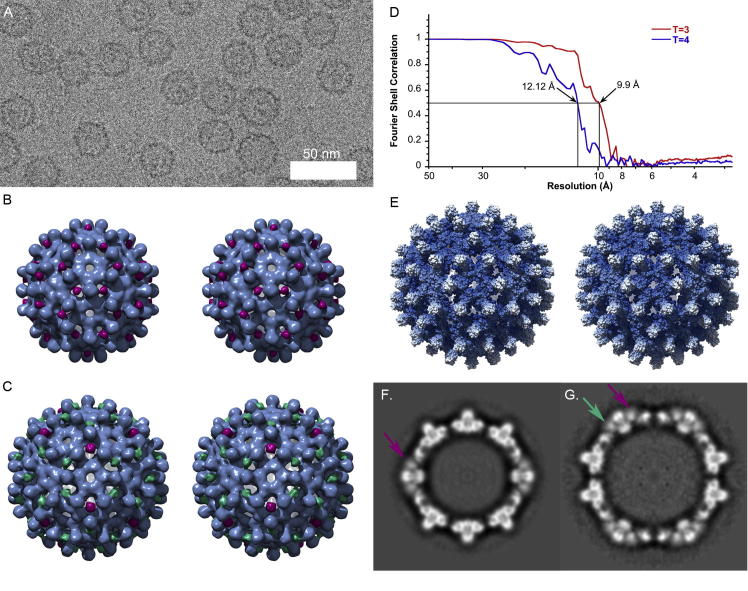
Fig.1.
3D reconstructions of His-β-L HBcAg virus-like particles. (A) Cryomicroscopy of His-β-L HBcAg VLPs revealed two sizes 30 nm T = 3 and 34 nm T = 4. (B) The T = 3 structure was generated using 6048 particle images and achieved a resolution of 10 Å. (C) The T = 4 VLP was reconstructed using 2040 particles and achieved a resolution of 12 Å. Resolution assessment was by the FSC0.5 criterion (D). The general architecture of both capsids is similar to the wild type HBcAg, as demonstrated by comparison with a solvent exclusion surface representation of the T = 4 capsid calculated from the X-ray structure PDB ID 1QGT (E). His-β-L HBcAg particles however were seen to bear an extra spike density hypothesised to comprise a trimer of the extended N-terminus, located at sites of local threefold symmetry in the T = 3 VLP (magenta) and at both icosahedral (green) and local (magenta) threefold symmetry axes in the T = 4 particle. Wall-eyed stereo pair images are presented (B, C and E). Cross-sections through the T = 3 (F) and T = 4 (G) reconstructions show that the density in the N-terminal spikes is blurred compared to that of the HBcAg component (magenta and green arrows).