Figure 3.
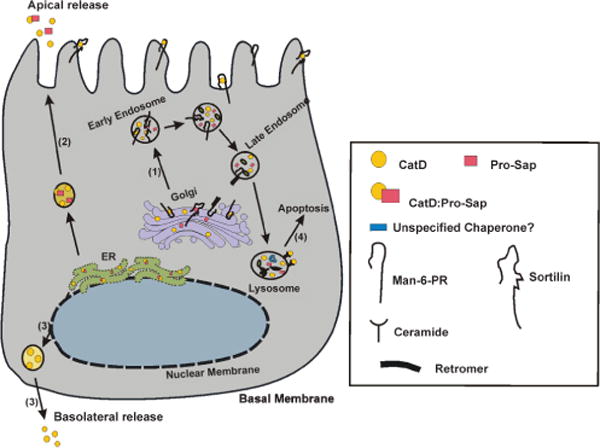
Model depicting different routes of CatD trafficking in association with its binding partners in polarized normal mammary epithelial cells, and their relevance to cancer: 1). When in the Golgi, the Man-6-P tagged CatD binds Man-6-PR (and/or sortilin) and is transported to the endosomal compartment. In the acidic environment, the complex is dissociated and Man-6-PR returns to the membrane with the retromer complex, while CatD is cleaved and processed in the late endosome and lysosomes. 2). Under normal conditions ≤5% of pro-CatD (either alone or in a complex with Pro-Sap) is secreted from the ER. 3). In polarized epithelial cells, the basolateral release of CatD is also observed, the binding partner in this case is unknown. However, in Caco-2 colon epithelial cell line, Man-6PR binds CatD and transports it basolateraly. 4). Generation of ceramide by acid sphingomyelinase results in the limited permeability of lysosomal membrane and release of CatD, leading to the induction of apoptosis. The majority of these pathways are altered in breast cancer. In pathway 1, the reduced acidification of endosomal/lysosomal compartment noted in cancer cells affects proper processing of CatD, resulting in increased secretion of pro-CatD. Routes 2 and 3 are greatly elevated and could lead to excessive ECM degradation. Route 4 could be equally affected by changes in vacuolar acidification, which alters CatD processing, its lysosomal release and participation in apoptosis.
