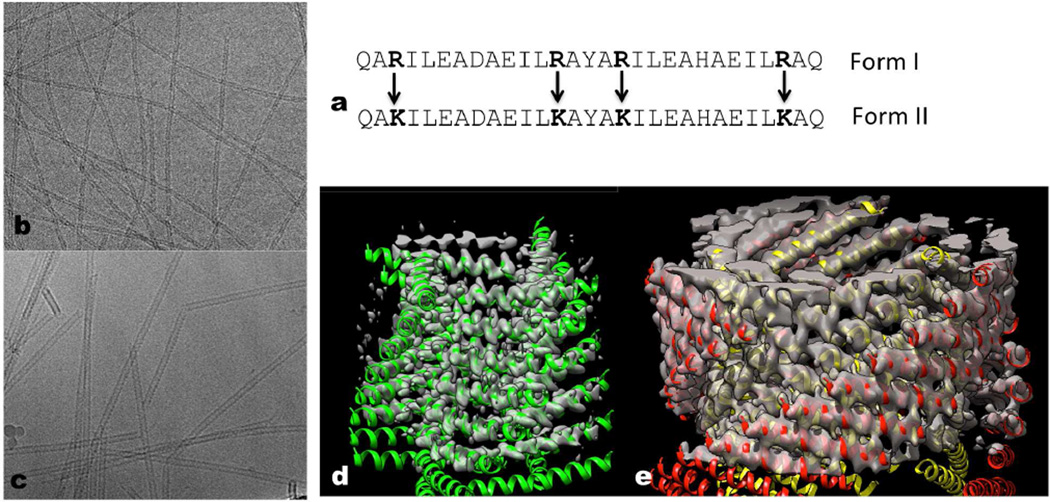
Figure 1. Conservative amino acid changes lead to two different helical forms.
a, The 29 residue peptide shown has four arginines, and this peptide polymerizes into a single-walled Form I polymer that has circa four subunits per turn. When the four arginines are changed to lysines, the peptides polymerize into a totally different Form II filament with circa three subunits per turn that is double-walled and has two peptides in each asymmetric unit. b–c, Electron cryo-micrographs of the Form I filament (b) (~ 60 Å diameter) and the Form II filament (c) (~ 120 Å diameter). d–e, Three-dimensional reconstructions of the Form I filament (d) and the Form II filament (e) have been fit with atomic models. In the Form II filament (e) there are two peptides in each asymmetric unit, and the outer helices have been colored red while the inner ones are yellow. In the Form I filament (d) each peptide is in an identical environment. The N-terminal region of the Form I helices (d) is melted, and density is not seen for this region at the high threshold used.