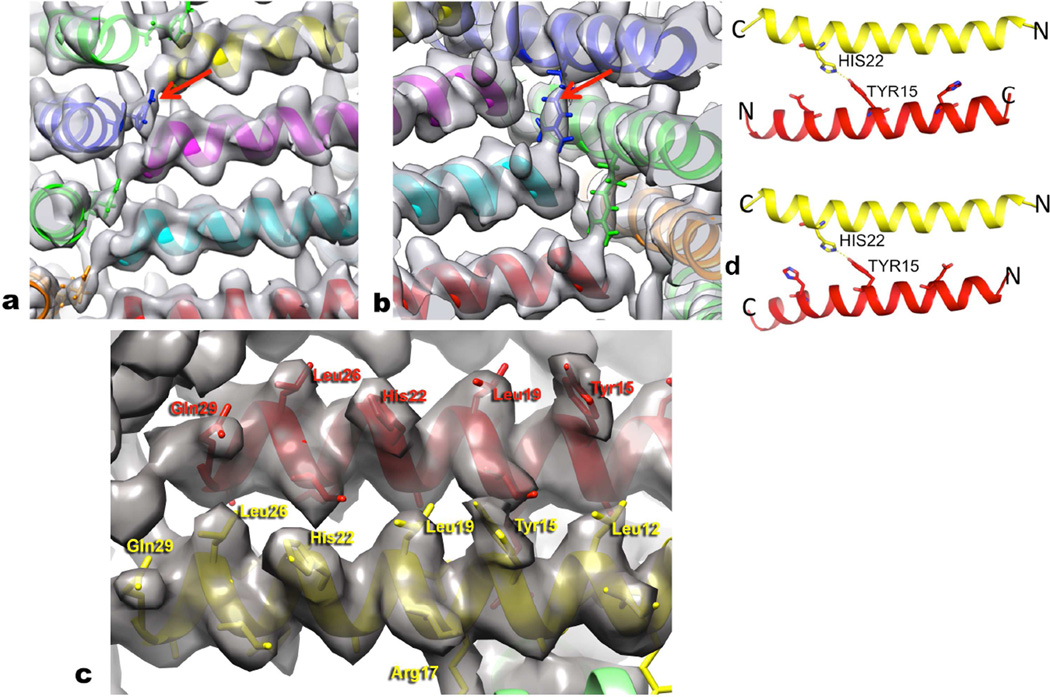
Figure 4. Details of the atomic models are testable.
a, In the Form I filament the single most ordered side chain is Arg13, which is sandwiched between two other subunits at every corner of the square as seen in this view from the outside of the filament. The Arg13 in blue (arrow) can be seen as tightly packed between the subunit in magenta and the one in yellow. b, Arg17 (arrow) provides a C-terminal cap for the α-helix formed by an adjacent peptide in Form I, and stabilizes the inside corners of the square. This view is from the lumen of the filament, and the Arg17 in blue extends to the C-terminus of the cyan helix. c, The quality and resolution of the Form I map can be seen when looking from the outside of the filaments. A number of the larger side chains have been labeled, and density exists to accommodate these residues. d, The lower resolution and nearly-palindromic nature of the sequence leads to ambiguity in the direction of the outer helix in the Form II assembly. In both models, parallel (bottom) and anti-parallel (top), His22 from the inner helix (yellow) interacts with Tyr15 in the outer helix (red). In both models, His22 from both the inner and outer helices is in the space between the two helices.