Abstract
Background
Increasing use of kidney grafts for simultaneous liver and kidney (SLK) transplants is causing concern about the most effective utilization of scarce kidney graft resources. This study evaluated the impact of implementing the proposed United Network for Organ Sharing (UNOS) SLK transplant policy on outcomes for end-stage liver disease (ESLD) and end-stage renal disease (ESRD) patients awaiting transplant.
Methods
A Markov model was constructed to simulate a hypothetical cohort of ESLD patients over a 30-year time horizon starting from age 50. The model applies the different criteria being considered in the UNOS policy and tallies outcomes, including numbers of procedures and life years after liver transplant alone (LTA) or SLK transplant.
Results
When1-week pre-transplant dialysis duration is required, the numbers of SLK transplants and LTAs would be 648 and 9,065, respectively. If the pre-transplant dialysis duration is extended to 12 weeks, there would be 240 SLK transplants and 9,426 LTAs. These change results in a decrease of 6,483 life years among SLK transplant recipients and an increase of 4,971 life years among LTA recipients. However, by increasing the dialysis duration to 12 weeks from 1 week, 408 kidney grafts would be released to the kidney waitlist due to the decline in SLK transplants; this yields 796 additional life years gained among ESRD patients.
Conclusion
Implementation of the proposed SLK transplant policy could restore access to kidney transplants for patients with ESRD albeit at the detriment of patients with ESLD and renal impairment.
Keywords: Simultaneous Liver and Kidney Transplantation, microsimulation, dialysis duration, end-stage liver disease
Introduction
Implementation of the Model for End-Stage Liver Disease (MELD) allocation system in the United States has prioritized transplantation in end-stage liver disease (ESLD) patients with renal impairment [1-2]. Survival after liver transplant alone (LTA) in individuals with renal impairment is poor [3-4]. Consequently, simultaneous liver and kidney (SLK) transplant, which affords improved survival compared to LTA in patients with irreversible renal impairment, has steadily increased [5-8]. However, there is great variation amongst transplant centers regarding the indications for SLK transplant.
United Network for Organ Sharing (UNOS) policy does not include listing requirements for SLK transplant candidates, but development of policy is currently underway [9]. Policy development has garnered much attention recently due both to a decline in post-SLK transplant survival over time and the deleterious effect of lengthening the kidney transplant queue with increasing SLK utilization [1]. Under the proposed policy, many individuals currently receiving SLK transplants would not be eligible. Briefly, the criteria recommend SLK listing for ESLD patients with: (1) chronic kidney disease stage 4/5; (2) acute renal failure with glomerular filtration rate (GFR) ≤25 mL/min/1.73 m2 for ≥6 weeks, and (3) metabolic disease such as hyperoxaluria [9]. Consequently, implementation of the proposed UNOS SLK policy might reduce the number of SLK transplants performed rendering more kidney grafts available to patients on the kidney wait list. Arguably, such a policy would have profound implications on patients with either cirrhosis or end-stage renal disease (ESRD) or both.
Simulation modeling has been widely employed to address different types of transplantation research questions, including graft allocation process, use of extended criteria donor graft vs. standard criteria donor graft, comparison of different transplant strategies, etc. [10-15]. However, simulation modeling has not been used to assess implementation of the proposed SLK transplant policy. Therefore, the objective of this study was to utilize simulation modeling to project the impact of implementing the proposed SLK policy on the net benefit/loss of life years for both ESLD and ESRD patients on their respective waitlists and to inform policy debates about how to best allocate limited number of kidney grafts.
Results
Incremental Gain/Loss in Life Years
Of 1,000,000 trials in the base case model, 935,059 LTAs and 32,580 SLK transplants (including 70,655 re-LTAs and 479 re-SLK transplants) were performed over the 30-year simulation period under the proposed SLK listing requirements. The proportion of the number of SLK transplants to the number of LTA was 3.49%. As the required pre-transplant dialysis duration increased from 1 week to 12 weeks for SLK transplant, the proportion of the number of SLK transplants to the number of LTA declined from 7.15% at 1-week to 2.54% at 12-weeks.
The total numbers of life years of ESLD patients on the waitlist undergoing LTA or SLK transplant from the one million trials over the 30-year simulation period in the base case were 16,831,550 and 525,579, respectively. Given that the range of actual annual number of LTA transplants in the OPTN/SRTR data from 1998 to 2007 was 9,538 - 11,126 per year, we calculated the number of life years gained/lost per 10,000 trials for LTA and SLK transplant. When the 1-week pre-transplant dialysis duration served as the baseline strategy and the 2-, 3-, 4-, 6-, and 12-week pre-transplant dialysis duration served as the comparator strategies, the incremental life years lost per 10,000 trials for SLK recipients was -2,623, -3,954, -4,278, -5,053, and -6,482, respectively. The incremental life years per 10,000 trials gained for LTA recipients was 2,823, 3,719, 3,686, 4,188, and 4,971, respectively. The total incremental life years gained/lost for LTA recipients and SLK transplant recipients due to implementation of stricter SLK listing requirements were 200, -236, -591, -865, and -1,512, respectively.
Meanwhile, the incremental number of kidney grafts released due to decrease in number of SLK transplant was 171, 255, 275, 322, and 408, respectively. Based on the OPTN/SRTR Data as of May 1, 2008, the difference in the remaining life expectancy between kidney transplant recipients and candidates on a waitlist ages 50-64 calculated by the Declining Exponential Approximation of Life Expectancy method (DEALE) was 1.95 years. Therefore, the incremental number of life years gained due to an increase in the number of kidney grafts to ESRD patients on the kidney waitlist was 333, 497, 537, 628, and 796, respectively. From this, the net benefit/loss of life years at 2-, 3-, 4-, 6-, and 12-week pre-transplant dialysis duration was 534, 262, -55, -237, and -715 life years, respectively (in Table 2).
Table 2. The numbers of life years of LTA and SLK transplant recipients under different durations of pre-transplant dialysis.
| Pre-transplant dialysis duration | 1 week | 2 weeks | 3 weeks | 4 weeks | 6 weeks | 12 weeks |
|---|---|---|---|---|---|---|
| Number of life years of LTA recipients | 164,128 | 166,951 | 167,847 | 167,814 | 168,316 | 169,099 |
| Incremental number of life years | 2,823 | 3,719 | 3,686 | 4,188 | 4,971 | |
| Number of life years of SLKrecipients | 10,309 | 7,686 | 6,354 | 6,031 | 5,256 | 3,826 |
| Incremental number of life years | −2,623 | -3,954 | −4,278 | −5,053 | −6,482 | |
| Sum of life years of LTA and SLK | 174,437 | 174,637 | 174,201 | 173,845 | 173,571 | 172,925 |
| Life years gained/lost among LTA and SLK | 200 | −236 | −591 | −865 | −1,512 | |
| Number of LTA cases | 9,065 | 9,234 | 9,296 | 9306 | 9,351 | 9426 |
| Number of SLK cases | 648 | 477 | 393 | 373 | 326 | 240 |
| Proportion of SLK to LTA | 7.15% | 5.17% | 4.23% | 4.01% | 3.49% | 2.54% |
| Number of kidney grafts released | 171 | 255 | 275 | 322 | 408 | |
| Life years gained per kidney1 | 1.95 | 1.95 | 1.95 | 1.95 | 1.95 | |
| Incr life years gained from ESRD patients | 333 | 497 | 537 | 628 | 796 | |
| Net benefit/loss of life years | 534 | 262 | −55 | −237 | −715 |
The difference in the remaining life expectancy between kidney transplant recipients and candidates on the waitlist ages 50-64, based on the OPTN/SRTR Data as of May 1, 2008
Sensitivity Analysis
Given the fact that dialysis might occur prior to transplant registration, we varied the pre-registry dialysis duration in the sensitivity analysis to assess impact of increase in pre-transplant dialysis duration on the model outcomes. The sensitivity analysis showed that the number of SLK transplants per 10,000 trials in the base case model has consistently increased from 326 to 456, as the pre-registry dialysis duration increased from 0 week to 12 weeks. In comparison with the 0-week pre-registry dialysis duration, additional number of kidney grafts consumed by SLK transplant increased from 46 at the 2-week pre-registry dialysis duration to 130 at the 12-week pre-registry dialysis duration. Concurrently, the proportion of the number of SLK transplants to the number of LTA increased from 3.49% to 4.93%. The incremental increase in the proportion of SLK transplant to LTA was 0.50%, 0.71%, 0.20% and 0.03%, as the pre-registry dialysis duration was serially extended from 0 to 2, 4, 6, and 12 weeks, respectively.
Model Validation
To validate the model outcomes, the projected survival rates in the base case model were compared observed survival rates in the OPTN/SRTR annual report and published literature targeting ESLD patients with renal impairment. For example, the projected 3-, 12-, 60-, and 120-month survival rates of LTA recipients with MELD score 21-30 and >30 in the model outcomes were 93.60% - 90.69%, 88.14% - 83.73%, 70.81% - 66.35%, and 55.18% - 49.71%, respectively. As compared to the observed corresponding survival rates in the OPTN/SRTR 2008 annual report (93.30% - 89.30%, 86.40% - 79.30%, 71.50% -68.00%, and 58.04% - 55.20%) [16], the differences in survival rates between the model outcomes and the observed data over the 10-year period were approximately ± 5%. The projected 12-, 24-, 36-month survival rates of SLK transplant recipients in the MELD score 21-30 and >30 categories from the model were 84.40% - 82.76%, 79.70% - 77.45%, and 75.24% - 72.08%, respectively, which was very close to the observed SLK survival rates (83.6%, 79%, and 74.8%) in the Gonwa' s study [3]. Thus, the model accurately projected the trend of survival rates for LTA recipients and SLK transplant recipients.
Discussion
This is the first study to use simulation modeling to assess the impact of implementing the proposed UNOS SLK transplant policy on both of ESLD patients with renal impairment and ESRD patients on each respective waitlist in the US. Overall, implementation of the proposed SLK transplant policy will lower number of SLK transplants and release kidney grafts to ESRD patients on kidney waitlist in the US. However, net loss of life years will occur when strict SLK transplant listing requirements are implemented. Finally, the pre-registry dialysis duration has a modest impact on increase in number of SLK transplants.
The model output showed that maximum net benefit of life years and net loss of life years occurred when the required pre-transplant dialysis duration was extended from 1 week to 2 weeks and ≥ 4 weeks, respectively. These findings were based upon survival advantage of kidney transplant recipients over candidates on waitlist calculated from the 2008 OPTN/SRTR annual report [16]. Therefore, whether implementation of strict SLK transplant listing requirements increased the net benefit in life years or not was contingent upon the difference between the incremental life years gained per KTA among kidney transplant recipients and the incremental life years lost among LTA recipients without renal recovery per kidney graft released due to LTA.
The pre-registry dialysis duration was not included in the UNOS/OPTN or SRTR dataset. As such, zero pre-registry dialysis duration was considered in the base case model. In the sensitivity analysis, we tested various durations of pre-registry dialysis and found that the pre-registry dialysis duration had modest effect on increase in number of SLK transplant. The peak in incremental increase in the proportion of SLK transplant to LTA occurred when the pre-registry dialysis duration was 4 weeks.
Transplant research is complex and involves many ethical and other challenges to conducting large population-based clinical trials to determine net outcomes. Accordingly, decision analysis and simulation modeling have been used to synthesize the best available data to inform clinical policy [17-18]. For example, investigators have used registration data to examine decline in renal function after liver transplant, and these risk prediction results have been validated and informed use of SLK transplant [19-22]. A recent decision analysis by Kiberd and colleagues [13] suggested that when both ESLD patients and ESRD patients are considered together, SLK transplantation does not maximize the total quality adjusted life years. Our study confirms that conclusion and extends Kiberd's results to identify the pre-transplant dialysis duration threshold that provides the best balance of the net benefit/loss of life years for both ESLD and ESRD patients, thereby providing new data that can be used by decision makers when developing policies for equitable and effective allocation of scarce kidney grafts.
An increase in SLK transplant as an unintended consequence of MELD-based liver allocation policy in the US has generated two competing patient populations (ESLD with renal impairment versus ESRD) for rare deceased donor kidney grafts. In Europe, the MELD allocation system, which was introduced in 2006, has led to a similar dilemma [23]. However, according to the Eurotransplant manual [24], SLK transplant has been excluded from the Approved Combined Organ policy, such that kidney grafts are not allocated preferentially with liver grafts according to medical urgency. Given that the MELD score does not adequately characterize the mortality associated with ESLD in the setting of chronic kidney disease, region-specific variances in allocation policy have been implemented. For instance, MELD exception points are granted to patients with polycystic liver and kidney disease in Germany when specific criteria are met (e.g., creatinine clearance ≤20-30 ml/min in combination with ascites or variceal bleeding). As such, SLK transplant still claims the largest proportion of combined organ transplants performed in Europe. Thus, how to allocate rare deceased kidney grafts remains a challenge to the worldwide transplant community.
This study has robust results, but the results should be considered in the context of several limitations. First, our study only considered use of standard criteria donor kidney grafts. This may limit the generalizability of our results. Second, our study did not consider quality of life in calculation of the differences in life years between kidney transplant recipients and candidates on the kidney waitlist. Net benefit from implementation of the proposed SLK transplant policy might be underestimated. Finally, the waiting time from addition to the waitlist to completion of transplant for each MELD quintile in this study was based on the median values in the U.S. national transplant registry. Given the need to match two organs, waiting list time could be longer for SLK transplant. This might result in overestimated number of SLK transplants in the model.
Conclusion
An unintended consequence of the implementation of MELD-based liver transplant allocation has been a dramatic increase in SLK transplant in the US. The current UNOS SLK transplant listing requirements may restore access to kidney transplant for patients with ESRD to the detriment of patients with ESLD and renal impairment. The proposed SLK policy underscore the dichotomy of life years lost for ESLD patients on the liver waitlist and life years gained for ESRD patients on the kidney waitlist.
Materials and Methods
Markov models have the ability to simulate all events of interest in a finite set of mutually exclusive health states and transitions between those states [18]. This type of model was utilized in this study to simulate ongoing risk (e.g., renal impairment) over time and repeat occurrence of an uncertain event (e.g., initiation or discontinuation of renal replacement therapy).
Model Overview
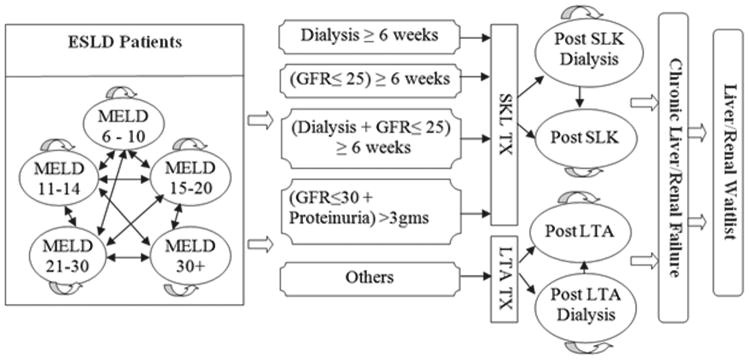
In this study, we constructed a discrete time state transition Markov model depicted in Figure 1 to simulate a hypothetical cohort of ESLD patients 50 years of age on a waitlist to undergo either LTA or SLK transplant, according to the proposed UNOS SLK policy. At the beginning of the simulation, transplant candidates were categorized into 10 health states based on the MELD score quintiles (6-10, 11-14, 15-20, 21-30, or >30) and dialysis status (yes or no). In addition, 5 interim dialysis states corresponding to each MELD score quintile were created to accommodate candidates who were transitioning among the dialysis states (yes or interim or no) to calculate an intermittent dialysis duration over the simulation period. When dialysis was suspended for greater than 3 weeks, the previous dialysis duration was changed to zero and the candidate entered the no dialysis state. In contrast, if the candidate returned to dialysis within 3 weeks, the previous dialysis duration was considered in the calculation of total dialysis duration. To accurately emulate the clinical situation, the model considers both the transition between MELD quintiles and changes in dialysis status. The eligible SLK transplant candidates in the base case model have received dialysis for 6 weeks prior to transplant. In the base case model, the LTA recipients who did not recover native renal function and SLK transplant recipients with neither kidney graft function nor native renal recovery by 3 months post-transplant were then placed on the kidney transplant waiting list. Chronic liver and/or kidney failure could occur post-transplant in either the LTA or SLK strategy. Liver and/or kidney re-transplant for acute and chronic graft failure were also considered in the model, despite the low likelihood of occurrence due to waitlist mortality, complication, etc. Tracker variables were used to record continuous or intermittent dialysis duration, waiting time, transplant counts (LTA, SLK, and kidney transplant alone (KTA)), waitlist mortality, duration of each GFR level in each MELD score quintile, number of LTA recipients who qualified for SLK transplant, and rate of renal function recovery. The Markov cycle length was 7 days, and a 30 year time horizon was selected as the simulation period. The model was constructed by using TreeAge Pro 2009 (TREEAGE Software Inc., Williamstown, MA, USA).
Simulation Method
Microsimulations were conducted to generate 1,000,000 trials for each scenario. A large number of microsimulations were conducted to ensure the stability of the results. The results of 1,000,000 trials in the model output were used to calculate survival rates for LTA or SLK transplant recipients. The number of life years within the waiting and post-transplant periods for LTA or SLK transplant recipients was calculated from the sum of survival period of each LTA or SLK transplant recipient over the 30-year simulation period. In calculation of incremental gain/loss in life years, numbers of LTA/SLK transplants per 10,000 trials were adopted, in accordance with actual annual number of liver transplants. The model outcomes were analyzed using STATA 11 (StataCorp LP, College Station, TX, USA).
Assumptions
The following assumptions were incorporated into the model due to limitations of the available data to derive transition probabilities. First, in the base case model, the same transplant probability was applied to both LTA and SLK transplant and varied according to MELD score quintile. Second, while duration of GFR ≤25 ml/min and proteinuria >3gms/day were among the criteria used in the proposed UNOS SLK transplant policy, survival rates of LTA recipients according to pre-transplant GFR or proteinuria levels were not available. Thus, we assumed survival of LTA recipients with a pre-transplant GFR ≤25 ml/min was identical to recipients with a pre-transplant serum creatinine (Cr) >2.0 mg/dL. For LTA recipients with a pre-transplant GFR >30 ml/min, post-transplant survival was assumed to be same as the unadjusted survival of LTA recipients in the Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR) annual report [16].
Validation
Validity of the model output was examined by comparison with data from the OPTN registry and published literature.
Data sources
The values and ranges of parameters in the model were obtained from (1) UNOS/OPTN data, (2) SRTR data, (3) United States Renal Data System (USRDS) data, (4) Northwestern University Enterprise Data Warehouse (EDW), and (5) published literature. Based on UNOS/OPTN data as of February 15, 2008, we estimated the initial dialysis prevalence rate, dialysis incidence rate, dialysis cessation rate, and dialysis recurrence rate for each MELD score quintile. For instance, the probability of dialysis cessation was determined from examination of the OPTN/UNOS data for the following dialysis histories: (1) candidates undergoing dialysis at registry and then stopping dialysis while on the waitlist, and (2) no dialysis at registration, but initiation of dialysis while on the waitlist with subsequent cessation of dialysis prior to transplant. GFR was estimated using the abbreviated MDRD formula: 186 ×[serum creatinine(mg/dL)]−1.154× [age] −0.203× [0.742 if patient is female]× [1.21 if patient is African-American] from the OPTN/UNOS and USRDS data [9]. The level of proteinuria and corresponding GFR values were estimated from the Northwestern University Enterprise Data Warehouse. Table 1 depicts the values and ranges of the parameter estimates used in the model.
Table 1. The values and ranges of the parameter estimates used in the model.
| Parameter Names | Base case | Range | Sources |
|---|---|---|---|
| Percentage of liver transplant candidates in MELD quintile | 16 | ||
|
|
|||
| MELD ≤ 10 | 43.1% | ||
|
|
|||
| 10 < MELD < 15 | 32.6% | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 20.0% | ||
|
|
|||
| 20 < MELD ≤ 30 | 3.6% | ||
|
|
|||
| 30 < MELD | 0.5% | ||
|
|
|||
| Time to liver transplant (days) | 16 | ||
|
|
|||
| MELD ≤ 10 | 589.31 | ||
|
|
|||
| 10 < MELD < 15 | 523.81 | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 303.4 | 261.8 – 329.32 | |
|
|
|||
| 20 < MELD ≤ 30 | 56 | 47.2 – 652 | |
|
|
|||
| 30 < MELD | 16.6 | 13.2 – 21.42 | |
|
|
|||
| Time to kidney transplant (days) | 1,326 | 16 | |
|
|
|||
| Annual Liver Waitlist Death Rates Per 1,000 Patient-Years | 16 | ||
|
|
|||
| MELD ≤ 10 | 33.7 | ||
|
|
|||
| 10 < MELD < 15 | 62.9 | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 141.6 | ||
|
|
|||
| 20 < MELD ≤ 30 | 707.4 | ||
|
|
|||
| 30 < MELD | 4,654 | ||
|
|
|||
| Annual Kidney Waitlist Death Rates Per 1,000 Patient-Years | 87.1 | 16 | |
|
|
|||
| Annual Dialysis Death Rates Per 1,000 Patient-Years | 133 | 27 | |
|
|
|||
| Prevalence of dialysis at registry | UNOS/OPTN data3 | ||
|
|
|||
| MELD ≤ 10 | 0.1% | ||
|
|
|||
| 10 < MELD < 15 | 0.1% | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 0.6% | ||
|
|
|||
| 20 < MELD ≤ 30 | 4.6% | ||
|
|
|||
| 30 < MELD | 9.5% | ||
|
|
|||
| Probability of starting dialysis on waitlist | UNOS/OPTN data3 | ||
|
|
|||
| MELD ≤ 10 | 0.4% | ||
|
|
|||
| 10 < MELD < 15 | 0.8% | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 1.8% | ||
|
|
|||
| 20 < MELD ≤ 30 | 3.8% | ||
|
|
|||
| 30 < MELD | 9.5% | ||
|
|
|||
| Probability of dialysiscessation on waitlist | UNOS/OPTN data3 | ||
|
|
|||
| MELD ≤ 10 | 0.2% | ||
|
|
|||
| 10 < MELD < 15 | 0.2% | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 0.5% | ||
|
|
|||
| 20 < MELD ≤ 30 | 3.4% | ||
|
|
|||
| 30 < MELD | 9.6% | ||
|
|
|||
| Probability of dialysis recurrence on waitlist | UNOS/OPTN data3 | ||
|
|
|||
| MELD ≤ 10 | 0.0% | ||
|
|
|||
| 10 < MELD < 15 | 0.0% | ||
|
|
|||
| 15 ≤ MELD ≤ 20 | 0.1% | ||
|
|
|||
| 20 < MELD ≤ 30 | 0.7% | ||
|
|
|||
| 30 < MELD | 1.5% | ||
|
|
|||
| Probability of liver transplant operative mortality | 3% | 28-30 | |
|
|
|||
| Probability of kidney transplant operative mortality | 1.3% | 31 | |
|
|
|||
| Probability of SLK transplant operative mortality | 4.3% | 28-31 | |
|
|
|||
| Probability of Proteinuria <= 3gm & GFR <= 25 in MELD > 30 | 90.9% | NMH4 | |
|
|
|||
| Probability of Proteinuria <= 3gm & GFR <= 25 in 20 < MELD ≤ 30 | 77.8% | NMH4 | |
|
|
|||
| Probability of dialysis and GFR > 30 in MELD > 30 | 36.6% | NMH4 | |
|
|
|||
| Probability of dialysis and GFR > 30 in 20 < MELD ≤ 30 | 14.3% | NMH4 | |
|
|
|||
| Probability of dialysis and GFR ≤ 25 in MELD > 30 | 76.9% | NMH4 | |
|
|
|||
| Probability of dialysis and GFR ≤ 25 in 20 < MELD ≤ 30 | 100% | NMH4 | |
|
|
|||
| Probability of no dialysis and GFR > 30 in MELD > 30 | 41.7% | NMH4 | |
|
|
|||
| Probability of no dialysis and GFR > 30 in 20 < MELD ≤ 30 | 90.7% | NMH4 | |
|
|
|||
| Probability of no dialysis and GFR > 30 in 15 ≤ MELD ≤ 20 | 100% | NMH4 | |
|
|
|||
| Probability of no dialysis and GFR > 30 in 10 < MELD < 15 | 100% | NMH4 | |
|
|
|||
| Probability of no dialysis and GFR > 30 in MELD ≤ 10 | 100% | NMH4 | |
|
|
|||
| Probability of proteinuria > 3 in 20 < MELD ≤ 30 | 36.4% | NMH4 | |
|
|
|||
| Probability of proteinuria > 3 in MELD > 30 | 35.3% | NMH4 | |
|
|
|||
| Probability of post LTA renal recovery, given pre-transplant RRT < 30 days | 70.8% | 26 | |
|
|
|||
| Probability of post LTA renal recovery, given 30 days < pre-transplant RRT < 60 days | 56.3% | 26 | |
|
|
|||
| Probability of post LTA renal recovery, given 60 days < pre-transplant RRT < 90 days | 23.5% | 26 | |
|
|
|||
| Probability of post LTA renal recovery, given pre-transplant RRT > 90 days3 | 11.5% | 26 | |
|
|
|||
| LTA recipient survival at 5 years,, when GFR ≥ 30 | 72% | 3 | |
|
|
|||
| LTA recipient survival at 5 years, when 25 < GFR < 30 | 67.5%5 | 3 | |
|
|
|||
| LTA recipient survival at 5 years, when GFR ≤ 25 | 63% | 3 | |
|
|
|||
| LTA recipient survival without renal recovery at 90 days | 64% | 26 | |
|
|
|||
| LTA recipient survival without renal recovery at 1500 days | 15% | 26 | |
|
|
|||
| SLK recipient survival at 5 years | 68% | 3 | |
|
|
|||
| Post SLK liver graft survival at 5 years | 62% | 32 | |
|
|
|||
| Post SLK kidney graft survival at 5 years | 60% | 32 | |
|
|
|||
| Post SLK renal graft survival at 3 months | 97.3% | NMH4 | |
|
|
|||
| Post LTA liver graft survival at 5 years, when GFR < 25 | 62% | 1, 32 | |
|
|
|||
| Post LTA kidney graft survival at 5 years, when GFR < 25 | 59% | 33 | |
|
|
|||
| Post LTA liver graft survival at 10 years, when GFR > 30 | 51.7% | 16 | |
|
|
|||
| Post LTA kidney graft survival at 3 years, when GFR > 30 | 86.1% | 34 | |
The number of waiting days was estimated from average of 25th percentile of time to transplant in 2002-2007
95% confidence interval of national median waiting time in 2003-2007
United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) data as of February 15, 2008
Northwestern University Enterprise Data Warehouse (EDW)
Survival of LTA recipients with 25 < GFR < 30 was obtained from average survival of LTA recipients with GFR ≥ 30 and GFR ≤ 25
The declining exponential function was used to convert the parameter values in the literature into weekly values. To convert rate to probability, the following equation was used: p=1-e-r*t where r=weekly rate; t= a week
Sensitivity analysis
Several studies have demonstrated that pre-transplant dialysis duration was the most critical parameter in predicting post-LTA native renal recovery [3-4, 25-26]. In our study, various durations of pre-transplant dialysis were used to select SLK transplant candidates.
In addition, pre-transplant dialysis duration was not accurately captured by either the UNOS/OPTN or SRTR datasets [16]. Calculation of dialysis duration in the base case model started on the transplant registration date, which might underestimate the true dialysis duration. Thus, the different pre-registration dialysis durations of 0 week, 2 weeks, 4 weeks, 6 weeks and 12 weeks were examined in the sensitivity analysis.
Figure 1.
Acknowledgments
The authors acknowledge Dr. Mandelblatt of Georgetown-Lombardi Cancer Center for assistance with manuscript review and editing.
Grants and financial support: This research was supported by the Cancer Intervention and Surveillance Modeling Network (CISNET) grant number U01 CA152958 from the National Cancer Institute and NIDRR grant number H133P080006 from the Department of Education
Abbreviation
- Cr
creatinine
- ESLD
end-stage liver disease
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate KTA, kidney transplant alone
- LTA
liver transplant alone
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- SLK
simultaneous liver and kidney
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
- USRDS
United States Renal Data System
Footnotes
- Yaojen Chang: Participated in construction and validation of simulation model, estimates of input parameters, interpretation of results, the writing of the paper
- Lorenzo Gallon: Participated in research design
- Kirti Shetty: Preparation of the final manuscript
- Yuchia Chang: Participated in data analysis
- Colleen Jay: Participated in research design
- Josh Levitsky: Participated in research design
- Bing Ho: Participated in research design
- Talia Baker: Participated in research design
- Daniela Ladner: Participated in research design
- John Friedewald: Participated in research design
- Michael Abecassis: Participated in research design
- Gordon Hazen: Participated in data analysis
- Anton I. Skaro: Participated in the performance of the research, preparation of the final manuscript
Conflict of interest: None
References
- 1.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008 Apr 15;85(7):935–42. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Galanko JA, Medapalli RK, Russo MW. Impact of dialysis and older age on survival after liver transplantation. Am J Transplant. 2006 Sep;6(9):2183–90. doi: 10.1111/j.1600-6143.2006.01454.x. Epub 2006 Jul 6. [DOI] [PubMed] [Google Scholar]
- 3.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006 Nov;6(11):2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 4.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002 May;35(5):1179–85. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 5.Papafragkakis H, Martin P, Akalin E. Combined liver and kidney transplantation. Curr Opin Organ Transplant. 2010 Jun;15(3):263–8. doi: 10.1097/MOT.0b013e328338f638. [DOI] [PubMed] [Google Scholar]
- 6.Hanish SI, Samaniego M, Mezrich JD, et al. Outcomes of Simultaneous Liver/Kidney Transplants Are Equivalent to Kidney Transplant Alone: A Preliminary Report. Transplantation. 2010 Jul 15;90(1):52–60. doi: 10.1097/tp.0b013e3181e17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra A, Cantarovich M, Bain VG. Simultaneous liver and kidney transplants: optimizing use of this double resource. Transplantation. 2011 Jun 27;91(12):1305–9. doi: 10.1097/TP.0b013e31821bad1b. [DOI] [PubMed] [Google Scholar]
- 8.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined Liver-Kidney Transplantation Is Preferable to Liver Transplant Alone for Cirrhotic Patients With Renal Failure. Transplantation. 2012 Aug 27;94(4):411–416. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Transplantation Committee and Liver and Intestinal Organ Transplantation Committee -Proposed listing requirements for simultaneous liver-kidney transplant candidates (Policy proposed: 3.5.10 - Simultaneous Liver-Kidney Transplantation) [accesses in Jan/2010]; http://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSub_237.pdf.
- 10.Thompson D, Waisanen L, Wolfe R, et al. Simulating the allocation of organs for transplantation. Health Care Manag Sci. 2004 Nov;7(4):331–8. doi: 10.1007/s10729-004-7541-3. [DOI] [PubMed] [Google Scholar]
- 11.Howard DH. Dynamic analysis of liver allocation policies. Med Decis Making. 2001 Jul-Aug;21(4):257–66. doi: 10.1177/0272989X0102100401. [DOI] [PubMed] [Google Scholar]
- 12.Amin MG1, Wolf MP, TenBrook JA, Jr, et al. Expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: a decision analysis. Liver Transpl. 2004 Dec;10(12):1468–75. doi: 10.1002/lt.20304. [DOI] [PubMed] [Google Scholar]
- 13.Kiberd B, Skedgel C, Alwayn I, Peltekian K. Simultaneous Liver Kidney Transplantation: A Medical Decision Analysis. Transplantation. 2011 Jan 15;91(1):121–127. doi: 10.1097/tp.0b013e3181fcc943. [DOI] [PubMed] [Google Scholar]
- 14.Jay CL, Skaro AI, Ladner DP, et al. Comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: Recognizing who can benefit. Liver Transpl. 2012 Jun;18(6):630–4. doi: 10.1002/lt.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale A, Volk ML, Gambato M, et al. Estimation of the harm to the waiting list as a crucial factor in the selection of patients with hepatocellular carcinoma for liver transplantation. Transplant Proc. 2010 May;42(4):1194–6. doi: 10.1016/j.transproceed.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 16.The 2008 OPTN/SRTR Annual Report: Transplant Data 1998-2007. [accessed in Jan/2012]; http://optn.transplant.hrsa.gov/ar2008/default.htm.
- 17.Siebert U, Alagoz O, Bayoumi AM, et al. ISPOR-SMDM Modeling Good Research Practices Task Force. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--3. Value Health. 2012 Sep-Oct;15(6):812–20. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993 Oct-Dec;13(4):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 19.Tanriover B, Mejia A, Weinstein J, et al. Analysis of kidney function and biopsy results in liver failure patients with renal dysfunction: a new look to combined liver kidney allocation in the post-MELD era. Transplantation. 2008 Dec 15;86(11):1548–53. doi: 10.1097/TP.0b013e31818b22cc. [DOI] [PubMed] [Google Scholar]
- 20.O'Riordan A, Donaldson N, Cairns H, et al. Risk score predicting decline in renal function postliver transplant: role in patient selection for combined liver kidney transplantation. Transplantation. 2010 Jun 15;89(11):1378–84. doi: 10.1097/TP.0b013e3181d9e195. [DOI] [PubMed] [Google Scholar]
- 21.Chopra Angeli, Cantarovich Marcelo, Bain VincentG. Simultaneous Liver and Kidney Transplants: Optimizing Use of This Double Resource. Transplantation. 2011 Jun 27;91(12):1305–1309. doi: 10.1097/TP.0b013e31821bad1b. [DOI] [PubMed] [Google Scholar]
- 22.Akkina SK1, Asrani SK, Peng Y, et al. Development of organ-specific donor risk indices. Liver Transpl. 2012 Apr;18(4):395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desschans B, Van Gelder F, Van Hees D, et al. Evolution in allocation rules for renal, hepatic, pancreatic and intestinal grafts. Acta Chir Belg. 2008 Jan-Feb;108(1):31–4. [PubMed] [Google Scholar]
- 24.Eurotransplant Manual. Chapter 5 ET Liver Allocation System (ELAS), version 4.3. 2014 Jan 13; [Google Scholar]
- 25.Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, Markmann JF, Olthoff KM, Shaked A, Reddy KR. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005 Sep;11(9):1048–55. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 26.Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010 Apr;16(4):440–6. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999 Dec 2;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999 Dec;30(6):1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 29.Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001 May;33(5):1080–6. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996 Mar 14;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth JM, Hollenbeck BK, Englesbe MJ, DeMonner S, Krein SL. Operative mortality after renal transplantation--does surgeon type matter? J Urol. 2007 Jun;177(6):2255–9. doi: 10.1016/j.juro.2007.02.006. discussion 2259. [DOI] [PubMed] [Google Scholar]
- 32.Pham PT, Pham PC, Wilkinson AH. Renal function outcomes following liver transplantation and combined liver-kidney transplantation. Nat Clin Pract Nephrol. 2007 Sep;3(9):507–14. doi: 10.1038/ncpneph0574. [DOI] [PubMed] [Google Scholar]
- 33.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003 Jul;9(7):741–7. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 34.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]


