Abstract
Objective
The aim of this study was to analyze the prognostic factors correlated with survival of patients with acute myeloid leukemia at the Hospital de Clínicas, Universidade Federal do Paraná between 2003 and 2009, as well as to investigate the clinical and epidemiological profile.
Methods
The overall survival and disease-free survival were statistically evaluated using the Kaplan–Meier method, the log-rank test and multivariate evaluation by Cox regression analysis.
Results
The study population was predominantly younger than 60 years old (81,6%), had intermediate cytogenetic risk (40.8%), in first complete remission after induction chemotherapy (46.9%), with a white blood count at diagnosis of less than 30 × 109/L (57.1%) and de novo acute myeloid leukemia (62.2%). Survival curves showed that better prognosis was related to age below 60 years (median:12,4 months; p-value = 0,2227; Odds Ratio = 0,6676), good prognostic cytogenetic markers (median: 97.7 months; p-value = 0.0037; Odds Ratio = 0.4239) and white blood cell count at diagnosis of less than 30 × 109/L (median survival: 23.6 months; p-value = 0.0001; Odds Ratio = 0.3651). Regarding the French-American-British subgroups, the median overall survival was 23.5 months for M0, M1 and M2, 97.7 months for M3 and 7.4 months for M4, M5, M6, and M7 (p-value = 0.0288).
Conclusion
Prognostic factors strongly influenced patient survival, as well as guided treatment. Moreover, these factors were consistent with the available literature adjusted for the population in question.
Keywords: Acute myeloid leukemia, Survival analysis, Prognosis, Adult, Cytogenetics
Introduction
Neoplastic diseases were historically associated with economically developed countries. For approximately four decades, however, this situation has changed and a lot of the onus is observed in developing countries. Infectious diseases are not the main cause of death anymore and cancer has acquired a greater dimension, becoming a global public health problem.1
Hematological malignancies represent 7% of new cancer cases each year.2 According to The Brazilian National Cancer Institute, it has been estimated that there were 4570 new cases in men and 3940 in women in 2012. Acute myeloid leukemia (AML) is the most common acute leukemia in adults and accounts for approximately 80% of the cases in this group, with an annual incidence of 2.7 cases per 100,000 population.3
AML is a relatively rare disease with high heterogeneity in the affected population in terms of morphology, immunophenotype, cytogenetics and molecular abnormalities. It is a clonal proliferation of myeloid precursors, the result of genetic and epigenetic alterations that disrupt self-renewal, proliferation and differentiation of cells, with accumulation of leukemic blasts or immature cells in the bone marrow.4 The clinical outcome is extremely variable, with survival from a few days to a definitive cure of some clinical and biological aspects, which is useful in predicting outcomes.4–6
Several clinical features can predict complete remission and the event-free survival (EFS) of these patients. The most important prognostic factors regarding adverse clinical presentations, include age, cytogenetic abnormalities, secondary leukemia, white blood cell (WBC) count and complete remission after the first induction.5,7
The cases can be morphologically subclassified according to the French-American-British (FAB) system. This form of organization does not provide additional prognostic information, but it is important to systematize acute promyelocytic leukemia, a biological and clinical variant of AML, classified as AML M3 in the FAB system, currently called acute promyelocytic leukemia with t(15,17)(q24.1,q21.1), and PML-RARA, in the World Health Organization (WHO) classification system.8–10
Brazil has peculiarities regarding its territorial dimensions, with important regional differences in the occurrence of the disease and distribution of associated risk factors and so local information is extremely important for analytical exploration of this malignancy.
Prospects for patients have improved over the last 30 years, but despite significant progress, the treatment outcome is variable and frequently suboptimal. More than half of young and adult patients, and about 90% of the deaths of over 60-year-old patients in this population are disease related.5,6,11,12
This study shows the indispensability of registries with standardized, up-to-date and representative information, due the considerable variations between populations in relation to survival and epidemiological characteristics which can predict treatment outcome.
The aim of this study was to analyze the influence of prognostic factors described in the literature correlated with survival of patients with acute myeloid leukemia treated between 2003 and 2009 at the Hematology and Oncology Service of the Hospital de Clínicas, Universidade Federal do Paraná (HC-UFPR), Brazil, as well as to trace the clinical and epidemiological profile of the patients.
Methods
This retrospective analytical study was conducted at HC-UFPR after being approved by the Ethics Committee of the hospital.
The study population was selected using records from the Computer Information Service and the Hospital Cancer Registry of HC-UFPR using the following inclusion criteria: the International Classification of Diseases (ICD) of AML, older than 15 years old and diagnosis between January 2003 and December 2009. The initial patient set consisted of patients, predominantly treated with combined induction chemotherapy using cytarabine and daunorubicin (the so-called “7 + 3″ regimen) for non-M3 leukemias, and all-trans retinoic acid (ATRA) alone or combined with an anthracycline for the M3 subtype.
Patients who were not treated exclusively in the Hematology and Oncology Service, HC-UFPR were excluded as were those who had biphenotypic leukemia, Fanconi anemia either associated with myelodysplastic syndrome or in isolation, those who were diagnosed before 2003 and those whose medical records were not available. The flowchart for selection of the study population is detailed in Figure 1.
Figure 1.
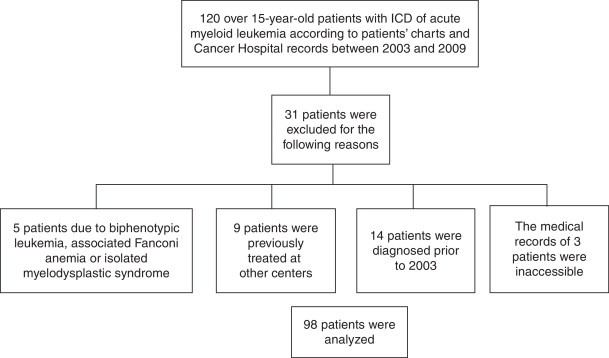
Flowchart used to select the study population.
Data collection was based on the review of medical records available from the Medical Archive Service (SAME), based on the results of cytogenetic and immunophenotyping examinations provided by the respective laboratories, as well as records from the Hospital Epidemiology Service of HC-UFPR. Information of interest was input on an Excel spreadsheet to facilitate further analysis of the variables and to compile the results.
Data for clinical and epidemiological characterization of the study population, such as gender, age at diagnosis, race, family history of cancer, cytogenetics, the presence of the t(15;17), complete remission rate after the first induction, WBC count at diagnosis and type of evolution (primary or secondary) were arranged in a table of absolute and relative frequencies, with calculations performed using the Microsoft Excel program.
Regarding the analysis of overall survival (OS) and EFS of the patients, survival curves were constructed by the Kaplan–Meier method, using the statistical program PRISM (version 5.0). The definitions used for the calculation of survival followed the revised recommendations of the International Working Group for therapeutic studies in acute myeloid leukemia.13 The OS was defined as the time interval between the date of diagnosis and date of death or date of last follow-up visit. The EFS was taken as the period between the date of diagnosis and the date of relapse, induction failure or date of death from any cause.
The curves of OS and EFS were also correlated with certain prognostic factors as reported in the literature, such as age at diagnosis, FAB classification, cytogenetics, WBC count at diagnosis and evolution (primary and secondary).11,12,14 A comparison of different curves was performed using the log-rank test and differences with p-value < 0.05 being considered statistically significant. The comparative analysis of the curves also included the Odds Ratio (OR) with respective 95% confidence intervals (95% CI).
Multivariate analyses of prognostic factors in relation to the OS, including cytogenetics, gender, age, WBC count and type of evolution were performed using Cox regression analysis with the SPSS 20.0 software.
The poor cytogenetic prognosis category included complex karyotypes (two or more unrelated abnormalities), monosomy of chromosome 5, monosomy of chromosome 7, translocations involving the 11q23 locus, and all deletions. The intermediate prognosis category was defined as normal karyotype and trisomies of chromosomes 4, 8 and 21. The translocations t(15;17), t(8;21) and inversions (16)/t(16;16) were considered the only entities capable of predicting a favorable prognosis. Other factors considered as poor prognosis in the current study were age greater than 60, a WBC count at diagnosis over 30 × 109/L, secondary leukemia and the lack of complete remission at first induction. Complete remission was considered to be the absence of signs and symptoms of disease associated with normal complete blood count and less than 5% blasts in bone marrow aspirate.
The categorization used was based on the French-American-British (FAB) group classification, which comprises eight subtypes of AML (M0–M7) and is based on morphological and cytochemical aspects.12 To compare survival curves, patients were divided into three groups: Group I (FAB M0–M2), Group II (FAB M3) and Group III (FAB M4–M7).
Results
The final study population was 98 individuals. Patients were predominantly younger than 60 years old (81.6%), in the intermediate cytogenetic risk group (40.8%), with first complete remission after induction chemotherapy (46.9%), white blood count less than 30 × 109/L (57.1%) and de novo leukemia (62.2%). The mean age at diagnosis was 44.27 years. All clinical and epidemiological features are listed in Table 1.
Table 1.
Global clinical and epidemiological characterization.
| n | % | |
|---|---|---|
| Gender | ||
| Female | 47 | 48 |
| Male | 51 | 52 |
| Age | ||
| 60 years or less | 80 | 81.6 |
| Over 60 years | 18 | 18.4 |
| Race | ||
| Caucasian | 91 | 92.9 |
| Non-Caucasian | 7 | 7.1 |
| Family cancer history | ||
| Present | 40 | 40.8 |
| Absent | 39 | 39.8 |
| Insufficient information | 19 | 19.4 |
| Cytogenetics | ||
| Good prognosis | 21 | 21.4 |
| Intermediary prognosis | 40 | 40.8 |
| Poor prognosis | 23 | 23.5 |
| Insufficient information | 14 | 14.3 |
| Translocation (15;17) | ||
| Present | 17 | 17.3 |
| Absent | 81 | 82.7 |
| Complete remission after first induction | ||
| Present | 46 | 46.9 |
| Absent | 43 | 43.9 |
| Insufficient information | 9 | 9.2 |
| White blood cell count | ||
| Lower than 30 × 109/L | 56 | 57.1 |
| Over than 30 × 109/L | 26 | 26.6 |
| Insufficient information | 16 | 16.3 |
| Type of leukemia | ||
| Primary | 61 | 62.2 |
| Secondary | 36 | 36.7 |
| Insufficient information | 1 | 1.1 |
There was no statistically significant difference when comparing the survival curves (Figure 2A) related to age for patients above and below the cut-off determined for the study population (median survival of 12.4 months for the group aged less than or equal to 60 years versus 8.2 months for the group older than 60 years; p-value = 0.2227; OR = 0.6676; 95% CI = 0.3488–1.278). The median EFS (Figure 2B) was 10.7 months for the younger group versus 7.3 months for the over 60-year olds (p-value = 0.2448; OR = 0.6812; 95% CI = 0.3567–1.301). The survival curve was not statistically significant for the gender-related SG with a median survival of 15.3 months for females versus 26.8 months for the men (p-value = 0.2756; OR = 0.7625; 95% CI = 0.4684–1.241).
Figure 2.
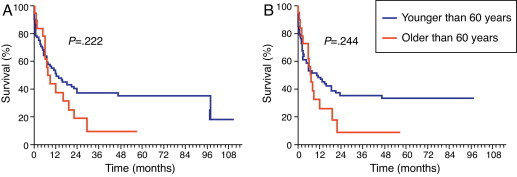
Age-related survival. (A) Overall survival and (B) event-free survival.
OS curves for the type of evolution did not show any statistically significant difference between the two groups with a median survival time for the primary leukemia group of 21.9 months versus 11.8 months for the group with secondary leukemia (p-value = 0.1706; OR = 0.6824; 95% CI = 0.3951–1.179).
This study found a significantly higher OS in the group with WBC count lower than 30 × 109/L at diagnosis (Figure 3A) with a median survival time of 23.6 months versus 4.7 months for the group with WBC count of more than 30 × 109/L at diagnosis (p-value = 0.0001; OR = 0.3651; 95% CI = 0.1403–0.5160). As for EFS (Figure 3B), the group with leukocyte counts lower than 30 × 109/L had a survival time of 19.3 months versus 3.0 months for the group with a leukocyte count of more than 30 × 109/L at diagnosis (p-value = 0.0003; OR = 0.3042; 95% CI = 0.1586–0.5834).
Figure 3.
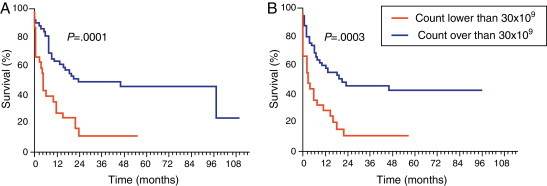
Survival in respect to white blood cell count. (A) Overall survival and (B) event-free survival.
There was a significantly longer OS for the subgroup in which cytogenetics was associated with good prognosis compared to the subgroup that included intermediate and poor prognosis (median survival time: 97.7 versus 9.2 months; p-value = 0.0037; OR = 0.4239; 95% CI = 0.2374–0.7568 – Figure 4A). A similar significance was observed for EFS (97.7 versus 6.3 months; p-value = 0.0005; OR = 0.3591; 95% CI = 0.2013–0.6407 – Figure 4B).
Figure 4.
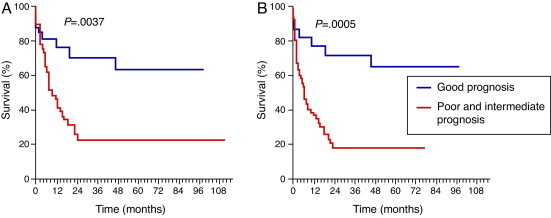
Survival in respect to cytogenetics. (A) Overall survival and (B) event-free survival.
Some services sent incomplete results for immunophenotyping and myelogram analysis and so only 86% of the results of the FAB classification were obtained. According to this classification, 5% of the patients were in the M0 subgroup, 1% in M1, 13% in M2, 31% in M3, 17% in M4, 11% in M5, 3% in M6 and 1% of the patients in M7. The M3 subgroup had statistically greater median OS and EFS as is shown in Figure 5A and B (median OS of 23.5 months for M0, M1, M2; 97.7 for M3 and 7.4 months for M4, M5, M6 and M7; p-value = 0.0288 and median EFS of 15.2 months for M0, M1, M2; 97.7 for M3 and 6.1 months for M4, M5, M6 and M7; p-value = 0.0047).
Figure 5.

Survival in respect to French-American-British classification. (A) Overall survival and (B) event-free survival.
Multivariate analysis was significant for cytogenetics (p-value = 0.015).
In this analysis, 65.3% of patients died; 25% of the deaths were due to disease progression. Bleeding was the second most common cause of death, followed by infection and the consequences of treatment.
Discussion
Information collected has significant relevance as there is no tradition of notification or any surveillance system for AML. Based on the absence of similar studies, we sought to gather data recorded in a referral service, HC-UFPR, during years to identify situations in which morbidity and mortality might be avoided. This study offers a fundamental methodological arsenal to understand health problems and to develop future analytical studies, despite the limitations inherent to retrospective studies.
The data shows a slight predominance of males than females and an absolute majority of self-identified Caucasian patients. Data available for Brazil is scarce, but data provided by the American Cancer Society show that AML is 1.7 times more prevalent in men and slightly higher in the non-Hispanic white population than other races.3 The high number of self-identified Caucasian patients may be related to the equally high prevalence of this race in the southern region of the country, where the research was conducted, or this is the kind of information provided by patients who, for historical and cultural reasons, tend to classify themselves as White.
The mean age at diagnosis was lower than that found in the literature.3,15 As there is no clear definition of the cut-off age to define the prognosis of AML, because of the statistical significance of the results, this study established the limit of 60 years to stratify the population into two groups. The disagreement with the literature regarding the statistical significance for this factor may be related to the incorporation of AML M3, prevalent in younger patients, with other subtypes of AML.3 The M3 subtype is a very peculiar form of leukemia and may be considered a particular disease, both by the clinical characteristics and by the good prognosis.9 However, due to the limited number of subjects, this subtype was grouped with other entities.
Older individuals had lower rates of complete remission, OS and EFS when compared with younger patients. AML in elderly is a biologically and clinically distinct entity. Based on the analysis of molecular and cytogenetic data, it is known that the leukemic cells in older patients are intrinsically resistant to conventional chemotherapy. Due to comorbidities and the poor reserve of stem cells in the bone marrow, older adults do not tolerate myelosuppressive chemotherapy well and there is a high treatment-related mortality rate and evolution of the disease.3,15,16
There was no statistical significance related to family history of cancer and the type of evolution (primary or secondary) of the disease in the prognosis of these patients. Familial occurrence is rare and its role in the development of the disease is uncertain, since the role of the type of evolution is well established in the literature. History of prior myelodysplasia or myeloproliferative diseases is common (24–40% of cases) in elderly patients with acute myeloid leukemia.17–19 The absence of any prognostic correlation may be related to the higher proportion of young patients in the study population.
Similar to the literature, the WBC count was found to be a significant prognostic factor in the univariate analysis. The limit of 30 × 109/L was adopted due to the higher statistical significance found for this value in the current study, although traditionally the decisive prognostic score is closer to 20 × 109/L.20
This study found a correlation between higher WBC counts and reduced OS and EFS. One possible explanation for this finding is that very high WBC counts are associated with an increased risk of tumor lysis syndrome and leukostasis. Both these are considered oncologic emergencies and are able to affect the prognosis of patients.21 In a multivariate analysis, this factor did not show the same significance, despite the borderline value (p-value = 0.063).
Cytogenetics is a key point in the diagnosis, treatment and prognosis of AML.22,23 In the present study, the survival curves of patients with cytogenetics related to poor prognosis and intermediate prognosis were grouped together with the prognosis being poor due to overlapping.
Cytogenetics was statistically significant both in univariate analysis and in multivariate analysis, showing that, irrespective of other factors, this is an important prognostic factor. Consistent with the literature, cytogenetics related to good prognosis conferred a better survival for patients.22–24 There was a significant drop in the survival curve of patients with cytogenetics related to poor prognosis; survival for this group was close to 20% within the first two years. The OS related to good prognosis became stable at between 70% and 80% in eight years of follow up. This data notoriously reveals the role of cytogenetics in the prognosis of patients.
Currently, the classification recommended for AML is one established by the WHO in 2008, which assesses the morphological, immunophenotypic and genetic combinations as well as clinical manifestations of the disease.7,25 Since this is a retrospective study and previous to WHO definitions, this study was based exclusively on the FAB classification associated with cytogenetics to establish subtypes.8
Acute promyelocytic leukemia (known as M3 in the FAB classification) is characterized by the translocation t(15;17) and is regarded as a particular type of AML.9 In the present study, similar to other publications, this kind of leukemia conferred better survival rates than those observed for the other subtypes.26–28
A higher death rate was observed related to the FAB M3 classification in the first days following diagnosis, probably due to the high frequency of complications, such as disseminated intravascular coagulation (characteristic of this class of AML). Subsequent deaths were mostly related to aggressive treatment. Moreover the survival curve shows that patients who survived the first stages of the illness and treatment had higher survival rates than those of other subgroups. We must stress that in this study the patients were grouped in respect to the FAB classification due to the overlap of the curves and the size of the population studied.
Conclusion
Prognostic factors significantly influenced the survival of patients, as well as guided treatment. Moreover, the outcomes were consistent with the literature, adjusted for the population in question. Clinical and epidemiological data provide important tools for a possible development of surveillance systems for AML, so that the necessities of the population consulted at HC-UFPR are prioritized and effectively treated by public health policies.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Estimativa 2012: incidência de câncer no Brasil [Internet]; 2011. Available from: www2.inca.gov.br/wps/wcm/connect/inca/portal/home [cited 30.6.12].
- 2.Guerra M.R., Moura Gallo C.V., Mendonça G.A. Risco de câncer no Brasil: tendências e estudos epidemiológicos mais recentes. Rev Bras Cancerol. 2005;51(3):227–234. [Google Scholar]
- 3.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Estey E.H. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88(4):318–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D. The changing paradigm of prognostic factors in acute myeloid leukaemia. Best Pract Res Clin Haematol. 2012;25(4):419–425. doi: 10.1016/j.beha.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Murati A., Brecqueville M., Devillier R., Mozziconacci M.J., Gelsi-Boyer V., Birnbaum D. Myeloid malignancies: mutations, models and management. BMC Cancer. 2012;12:304. doi: 10.1186/1471-2407-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekeres M.A., Peterson B., Dodge R.K., Mayer R.J., Moore J.O., Lee E.J. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood. 2004;103(11):4036–4042. doi: 10.1182/blood-2003-09-3118. [DOI] [PubMed] [Google Scholar]
- 8.Bennett J.M., Catovsky D., Daniel M.T., Flandrin G., Galton D.A., Gralnick H.R. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103(4):620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 9.Warrell R.P., de Thé H., Wang Z.Y., Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H. IARC Press; 2008. The World Health Organization classification of tumors of hematopoietic and lymphoid tissues. [Google Scholar]
- 11.Ferrara F., Schiff C.A. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 12.Liesveld J. Management of AML: who do we really cure? Leuk Res. 2012;36(12):1475–1480. doi: 10.1016/j.leukres.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Cheson B.D., Bennett J.M., Kopecky K.J., Büchner T., Willman C.L., Estey E.H. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Souto Filho J.T., Portugal R.D., Loureiro M., Pulcheri W., Nucci M. Characterization and analysis of the outcome of adults with acute myeloid leukemia treated in a Brazilian University hospital over three decades. Braz J Med Biol Res. 2011;44(7):660–665. doi: 10.1590/s0100-879x2011007500058. [DOI] [PubMed] [Google Scholar]
- 15.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25(14):1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 16.Stone R.M. The difficult problem of acute myeloid leukemia in the older adult. CA Cancer J Clin. 2002;52(6):363–371. doi: 10.3322/canjclin.52.6.363. [DOI] [PubMed] [Google Scholar]
- 17.Owen C., Barnett M., Fitzgibbon J. Familial myelodysplasia and acute myeloid leukaemia – a review. Br J Haematol. 2008;140(2):123–132. doi: 10.1111/j.1365-2141.2007.06909.x. [DOI] [PubMed] [Google Scholar]
- 18.Yagasaki H., Mugishima H. [Hereditary diseases with propensity to myeloid malignancy] Nihon Rinsho. 2009;67(10):1884–1888. [PubMed] [Google Scholar]
- 19.Wahlin A., Markevärn B., Golovleva I., Nilsson M. Prognostic significance of risk group stratification in elderly patients with acute myeloid leukaemia. Br J Haematol. 2001;115(1):25–33. doi: 10.1046/j.1365-2141.2001.03043.x. [DOI] [PubMed] [Google Scholar]
- 20.Su L., Li W., Cui J.W., Tan Y.H., Yang Y., Liu X.L. [Correlation of NPM1, FLT3-ITD mutations with leukocyte count and myeloblasts percentage in AML patients with normal karyotype] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(3):571–575. doi: 10.7534/j.issn.1009-2137.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.van Buchem M.A., te Velde J., Willemze R., Spaander P.J. Leucostasis, an underestimated cause of death in leukaemia. Blut. 1988;56(1):39–44. doi: 10.1007/BF00321058. [DOI] [PubMed] [Google Scholar]
- 22.Mrózek K., Marcucci G., Nicolet D., Maharry K.S., Becker H., Whitman S.P. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimwade D., Hills R.K., Moorman A.V., Walker H., Chatters S., Goldstone A.H. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 24.Döhner H., Estey E.H., Amadori S., Appelbaum F.R., Büchner T., Burnett A.K. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 25.Vardiman J.W., Thiele J., Arber D.A., Brunning R.D., Borowitz M.J., Porwit A. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 26.Asou N., Adachi K., Tamura J., Kanamaru A., Kageyama S., Hiraoka A. Analysis of prognostic factors in newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Japan Adult Leukemia Study Group. J Clin Oncol. 1998;16(1):78–85. doi: 10.1200/JCO.1998.16.1.78. [DOI] [PubMed] [Google Scholar]
- 27.Hiorns L.R., Swansbury G.J., Mehta J., Min T., Dainton M.G., Treleaven J. Additional chromosome abnormalities confer worse prognosis in acute promyelocytic leukaemia. Br J Haematol. 1997;96(2):314–321. doi: 10.1046/j.1365-2141.1997.d01-2037.x. [DOI] [PubMed] [Google Scholar]
- 28.Grimwade D., Walker H., Oliver F., Wheatley K., Harrison C., Harrison G. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]


