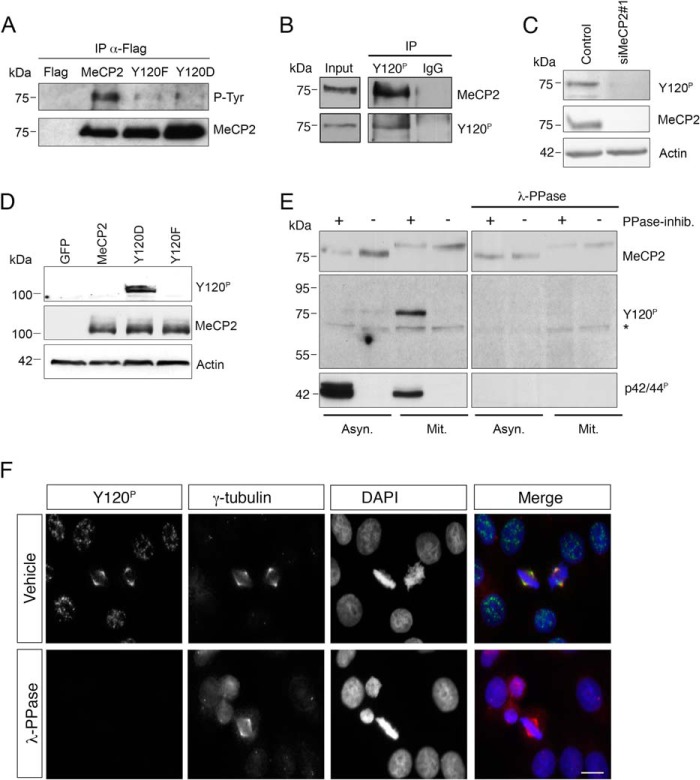
FIGURE 1.
MeCP2 phosphorylated at Tyr-120 is localized at the centrosome. A, exogenously expressed FLAG-tagged MeCP2 or the Y120F and Y120D derivatives were immunoprecipitated (IP) from HEK293 cell extracts with anti-FLAG antibodies, and the immunocomplexes analyzed by WB for phosphorylated tyrosine (P-Tyr) and MeCP2. Expression of the empty FLAG vector was used as negative control (n = 3). B, a HeLa whole cell extract was used for immunoprecipitation with the phospho-specific MeCP2 Tyr(P)-120 antibody or, as a negative control, with rabbit IgG. The presence of MeCP2 or MeCP2 Tyr(P)-120 in the immunocomplexes was then confirmed by WB with the indicated antibodies. The input corresponds to 0.5% of the total extract used for the immunoprecipitation. (n = 2). C, HeLa cells were transfected with siMeCP2#1 or with the corresponding control siRNA. Four days post-transfection, the levels of MeCP2 and Tyr(P)-120 were analyzed by WB, using actin as loading control (n = 3). D, wild-type MeCP2 or the Y120F and Y120D derivatives were expressed in HeLa cells as GFP fusion proteins and analyzed by WB with antibodies against MeCP2 Tyr(P)-120 or total MeCP2. Actin was used as loading control. E, extracts were prepared from asynchronous (Asyn) or mitotic (Mit) HeLa cells with or without phosphatase inhibitors (PPase-inhib.) and separated by SDS-PAGE. Proteins were transferred to a nitrocellulose membrane that was treated with λ-PPase for 16 h or left untreated. WB was performed with the indicated antibodies, using anti-p42/44P (pERK) as a control for the PPase treatment. The asterisk indicates a nonspecific band. F, HeLa cells were grown on coverslips, fixed, and treated with λ-PPase for 1 h or left untreated before immunostaining with antibodies against MeCP2 Tyr(P)-120 (green) and γ-tubulin (red). DNA was stained with DAPI (blue) (n = 3). Scale bar = 10 μm.