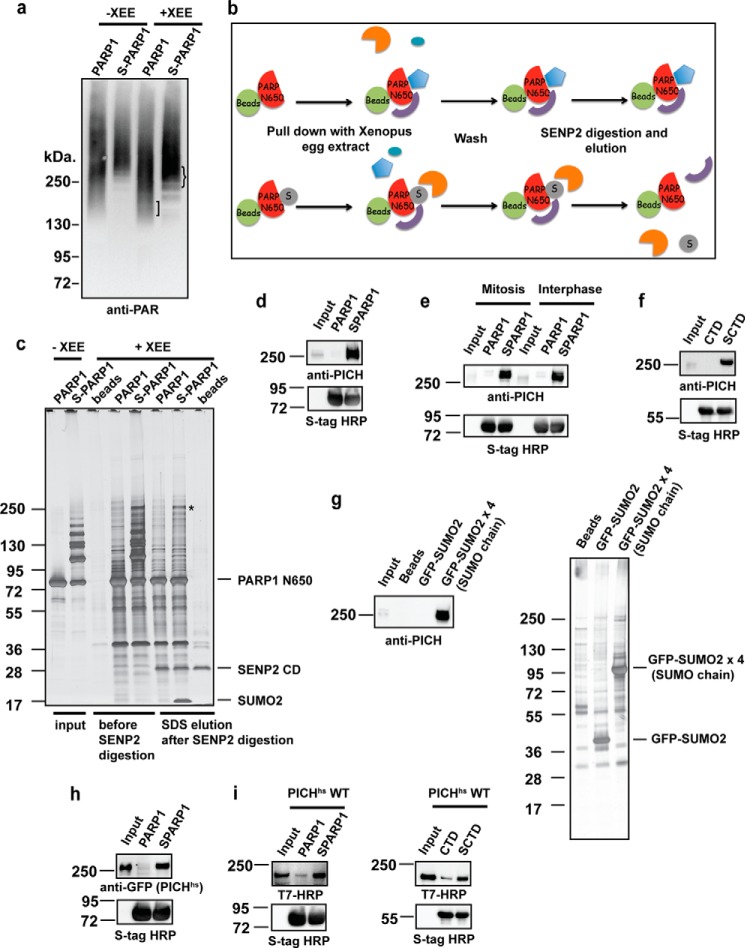
FIGURE 1.
PICH (also known as ERCC6-like) interacts with SUMOylated substrates. a, differences in the PARylation profiles of PARP1 (PARP1) and SUMOylated PARP1 (S-PARP1) pulled down samples from XEE. The PARylation profile of pulled down samples was analyzed as described under “Experimental Procedures.” The baits are indicated on each lane, and lanes 1 and 2 were not incubated with XEE, showing the self-PARylation of PARP1 and S-PARP1, respectively. b, schematic diagram of the pulldown assay. PARP1/TopoIIα-CTD and SUMOylated PARP1/TopoIIα-CTD (SCTD) were bound to S-protein-agarose beads. After incubation with XEE and washes, the bound proteins were incubated with the catalytic domain of SENP2. SENP2 assisted in the elimination of SUMOylated PARP1/TopoIIα-CTD bands during the analysis by deconjugating SUMO. c, isolation of SUMOylated PARP1-specific binding proteins. A pulldown assay was performed with the non-SUMOylated (PARP1) and SUMOylated PARP1-N650 (S-PARP1) fragment, which were bound to S-agarose beads. Bound proteins were visualized by silver staining. SENP2 digestion eliminated the SUMOylated PARP1-N650 bands. A 250-kDa protein that specifically interacted with S-PARP1 (indicated by an asterisk) was identified as PICH by LC-MS/MS. d, specific interaction of PICH with SUMOylated PARP1-N650. Pulldown fractions digested by SENP2 as in c were subjected to immunoblotting using an affinity-purified anti-PICH antibody. The CSF input lane on the membrane was ∼3% of the XEE used for the assay. The amount of bait (PARP1-N650) was analyzed by immunoblotting using S-tag HRP. e, cell cycle-independent PICH interaction with SUMOylated substrates. PICH interaction with SUMOylated PARP1-N650 was analyzed by pulldown assays using mitotic or interphase XEE. S-tag HRP was used to detect the amount of bait. f, PICH interacts with multiple SUMOylated substrates. Pulldown assays were performed with non-SUMOylated and the SUMOylated TopoIIα CTD (SCTD). SENP2 eluted fractions were analyzed by immunoblotting using an anti-PICH antibody. S-tag HRP was used as the bait loading control after SENP2 digestion. g, PICH interacts with SUMO chain. A pulldown assay was performed with GFP-SUMO and GFP-SUMO chain. The pulled down fractions were analyzed by silver staining and immunoblotting using an anti-PICH antibody. h, PICH-SUMO interaction is conserved in humans. XEE expressing EGFP-tagged PICHhs was subjected to pulldown assays. PICHhs in pulldown fractions was analyzed by immunoblotting with an anti-GFP antibody. S-tag HRP depicted the amount of bait in each sample. I, PICHhs directly interacts with SUMOylated PARP1-N650 and CTD. Purified recombinant PICHhs fused to a T7 tag was incubated with non-SUMOylated and SUMOylated PARP1-N650 or TopoIIα-CTD. The bound fraction was analyzed by immunoblotting using an anti-T7 tag-HRP antibody.