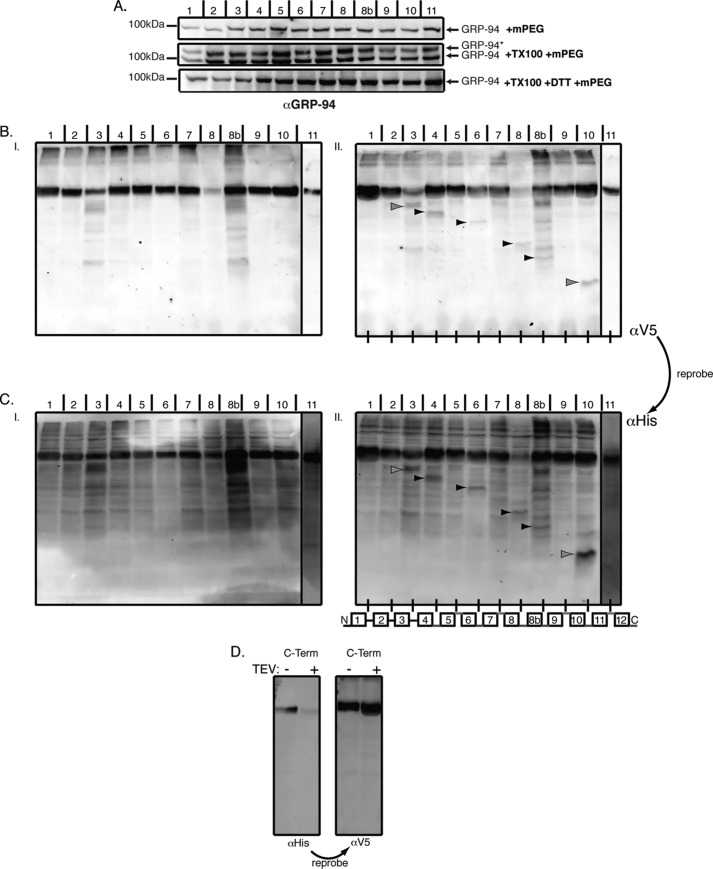
FIGURE 2.
Mapping of HHAT topology by TEV cleavage of microsomal preparations. HHAT constructs containing a C-terminal V5-His6 epitope tag and an internal TEV protease tag at the indicated loops were transfected into HEK293a cells, and intact microsomal preparations were prepared by ultracentrifugation. A, to show that the microsomal preparations have intact ER membranes, mPEG, a hydrophilic reagent of 5 kDa that reacts with -SH groups, did not react with the non-disulfide-bonded cysteine in the ER luminal protein GRP94 when it was added to 10 μg of the microsomal preparations (top panel). After treatment of the microsomes with 0.2% Triton X-100 (TX100, center panel), GRP94 is modified by mPEG, and the reaction could be blocked by the reducing agent DTT (bottom panel). GRP94* indicates the position of the modified proteins. B, microsome preparations were treated with TEV buffer (I) or Pro-TEV protease (see “Experimental Procedures”) (II) for 4 h at 25 °C with agitation. The reaction was then stopped with sample buffer, and samples were analyzed by SDS-PAGE and probed first with anti-V5 antibody and then reprobed with anti-His6 antibody. Because the microsomal preparations are intact, any TEV cleavage sites on luminal loops will be inaccessible to the Pro-TEV protease. However, any cytosolic sites will be accessible to the protease, and so the protein will be cleaved and produce a shorter C-terminal fragment that will be identified by antibody labeling. C, using this method, the HHAT topology model (II, bottom panel) was produced. Red lines indicate that the TEV site was inaccessible and, hence, luminal. Blue lines indicate that the TEV site was accessible and, hence, cytoplasmic. Cleaved bands are indicated by black arrowheads, whereas gray arrowheads indicate potential cleaved products. It was unclear whether mutants 1/2 and 2/3 produced any cleavage product. Immunoblots shown are representative results from five independent experiments. D, a construct containing the TEV protease tag in the linker sequence between the C-terminal (C-term.) V5 epitope and the His6 epitope tag was used to examine the topology of the C terminus of HHAT. Specifically, if the C terminus is cytosolic and accessible to TEV protease cleavage, then reactivity of the protein with a His6 antibody would be lost after treatment with Pro-TEV, whereas reactivity with the V5 antibody should be retained. This is what was observed in the experiments with this construct, indicating that the C terminus is cytosolic.