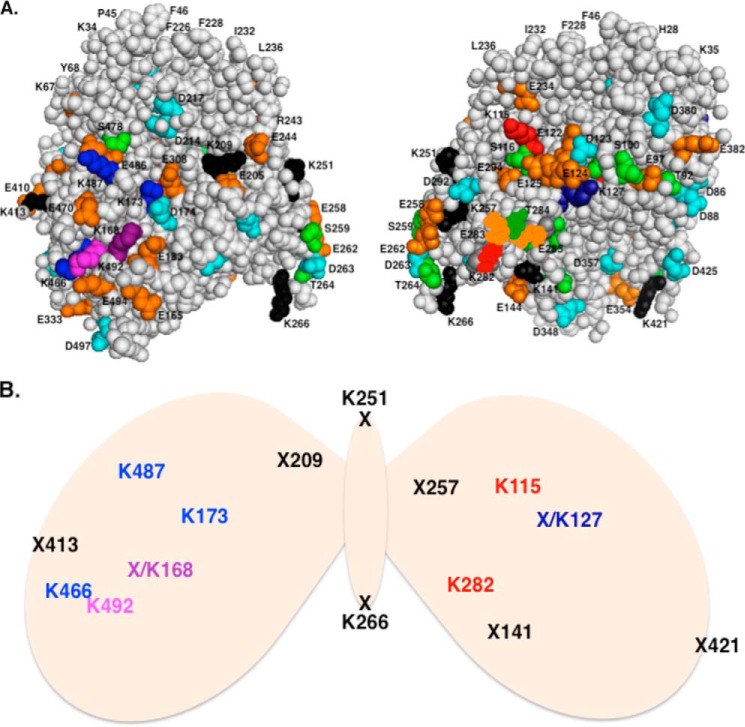
FIGURE 4.
CYP3A4 phosphorylation, ubiquitination, and cross-linking sites. A, two diametrically opposite external CYP3A4 structural surfaces splayed on either side of its Glu-258–Ser-259–Glu-262–Asp-263–Thr-264–Lys-266 acidic cluster are shown (top). Previously identified Ser/Thr phosphorylation sites (38) are shown in green, Asp residues in cyan, and Glu residues in orange. Lys residues ubiquitinated by UBC7-gp78 are shown in red, by CHIP complex in blue, and by both E2-E3 systems in magenta. Lys residues found to be cross-linked to either E2-E3 system are in black. B, butterflied CYP3A4 structure depicting cross-linked and/or ubiquitinated residues in a somewhat symmetrical pattern on each diametrically opposite CYP3A4 external surface are schematically illustrated. In addition to the Lys sites ubiquitinated by CHIP (blue), gp78 (red), or both (magenta), Lys residues that are cross-linked are shown as X. The known CYP3A4 K-ubiquitination sites that are also cross-linked (X/K) are shown in deep blue or deep purple.