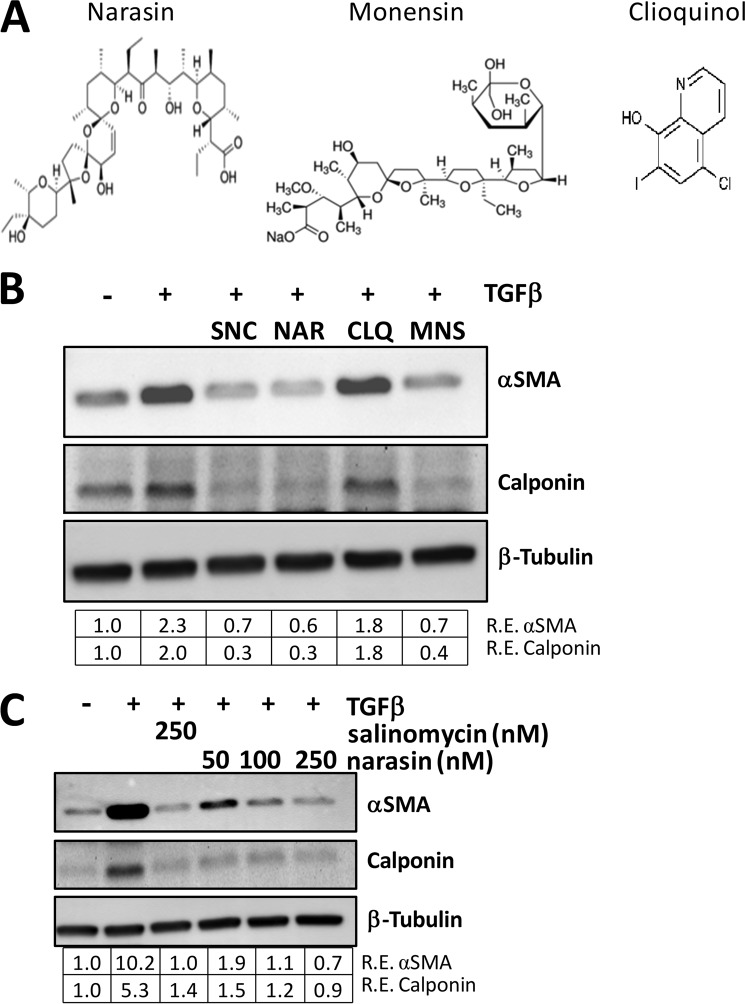
FIGURE 4.
The polyether ionophores salinomycin, narasin, and monensin inhibit myofibroblast formation. A, molecular structures of the ionophores narasin, monensin, and clioquinol. Narasin, a methylated derivative of salinomycin, and monensin are polyether ionophores, whereas clioquinol is an unrelated ionophore. B, human fibroblasts were treated with vehicle (DMSO), TGFβ, or TGFβ plus 250 nm of the indicated compounds for 72 h and then analyzed for myofibroblast markers by Western blot. Salinomycin (SNC), narasin (NAR), and monensin (MNS) all inhibited expression of αSMA and calponin, whereas clioquinol (CLQ) did not. Relative expression (R.E.) of αSMA and calponin (both normalized to β-tubulin) as determined by densitometry is shown below each lane. C, human fibroblasts were treated with 10–250 nm narasin or 250 nm salinomycin and TGFβ (1 ng/ml) for 72 h, and then cells were isolated and analyzed as in B. Salinomycin (250 nm) inhibited expression of αSMA and calponin 10- and 5-fold, respectively, whereas narasin exhibited a dose-dependent decrease in expression of the myofibroblast markers, where 250 nm narasin inhibited expression of αSMA and calponin by more than 10- and 5-fold, respectively. Experiments were repeated in two different strains, with representative results shown.