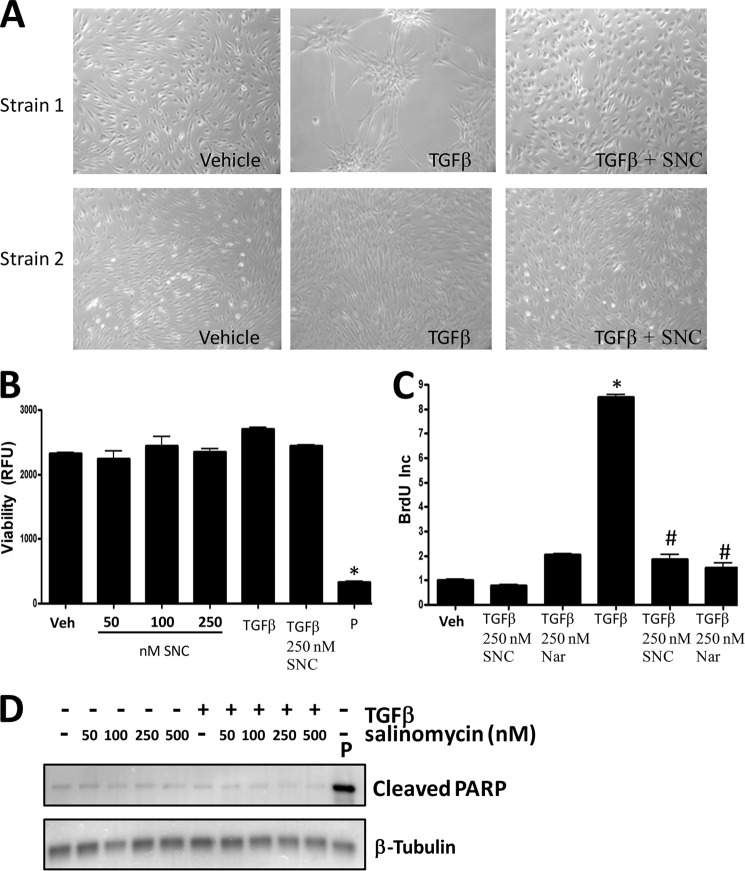
FIGURE 6.
Salinomycin does not affect viability or basal proliferation of human fibroblasts but does block TGFβ-induced proliferation. A, human fibroblasts were treated with vehicle (DMSO), TGFβ (1 ng/ml), or TGFβ plus 250 nm salinomycin (SNC) and cultured for 72 h. After culture, cell images were taken (original magnification ×200). Two different primary fibroblast strains at two different culture confluence states are shown. TGFβ induces morphology changes in human fibroblasts, indicative of myofibroblast formation. Cotreatment with salinomycin effectively blocked the morphological changes induced by TGFβ, and fibroblasts appeared similar to vehicle-treated fibroblasts. B, human fibroblasts were treated with vehicle (Veh, DMSO); 50, 100, or 250 nm salinomycin; TGFβ (1 ng/ml); and TGFβ plus 250 nm salinomycin or puromycin (P) (5 μg/ml) for 72 h in the presence of the redox-sensitive fluorescent dye Alamar Blue. After 72 h, fluorescence was measured to analyze cellular viability. RFU, relative fluorescent units. Puromycin, which served as a positive control, resulted in a total loss of cell viability, whereas salinomycin did not significantly affect cell viability at the tested doses either in the presence or absence of TGFβ. C, human fibroblasts were treated with vehicle (DMSO), 250 nm salinomycin, 250 nm narasin (Nar), TGFβ alone, or TGFβ plus salinomycin or narasin (250 nm) for 24 h before addition of BrdU. BrdU treatment was carried out for an additional 24 h, and then cells were fixed and stained for BrdU incorporation, which served as a measure of cell proliferation. Salinomycin and narasin did not affect basal fibroblast proliferation (first three columns). However, salinomycin and narasin significantly blocked TGFβ-induced proliferation (last three columns). Results are from a representative experiment performed in triplicate. *, p < 0.01 versus vehicle; #, p < 0.01 versus TGFβ treatment. D, primary human fibroblasts were treated with salinomycin (50–500 nm) in the presence or absence of TGFβ (1 ng/ml) for 72 h, and then cell extracts were collected and analyzed by Western blot for the apoptotic marker cleaved PARP. β-tubulin was used as a loading control. Cells were treated with puromycin as a positive control to induce apoptosis. As expected, puromycin induced cleaved PARP levels. However, salinomycin at the doses used (50–500 nm) did not induce PARP cleavage.