FIGURE 7.
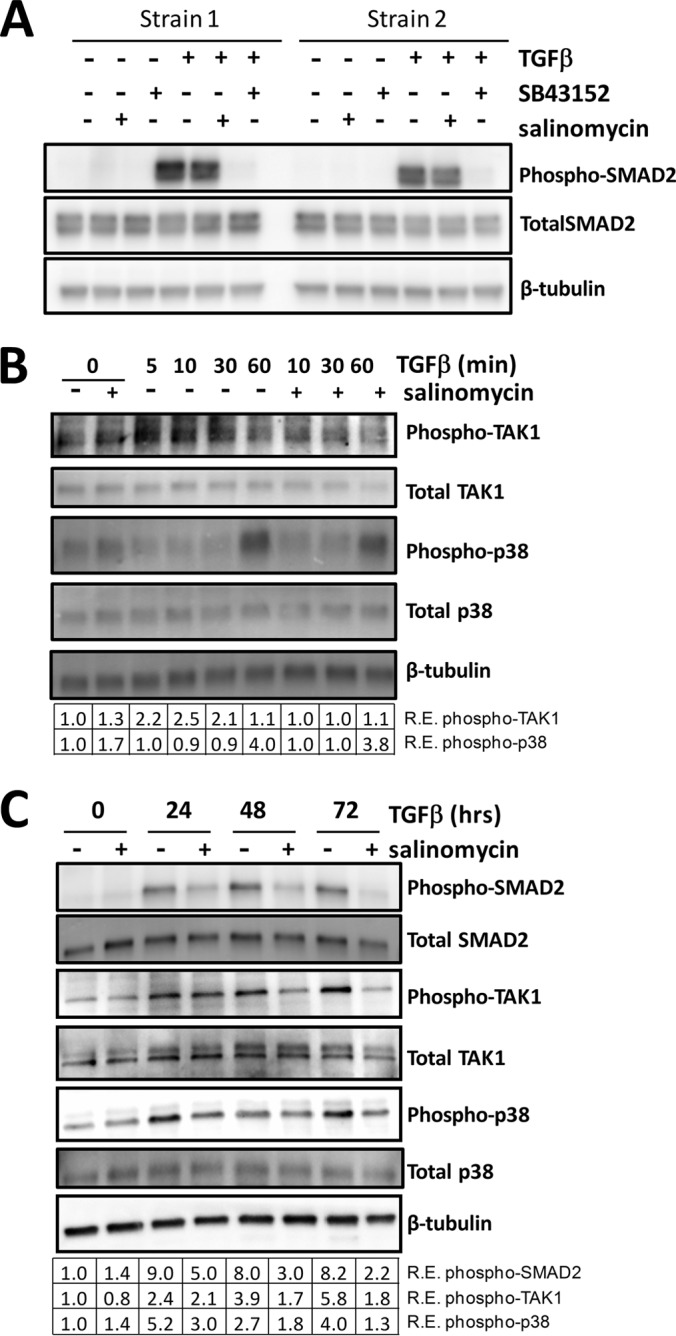
Salinomycin does not directly target TGFβ-induced SMAD2 phosphorylation but inhibits TGFβ-induced TAK1 and p38 phosphorylation. A, human fibroblasts were treated with vehicle, salinomycin, or SB-43152 with or without TGFβ. Cells were harvested at 1 h and analyzed for phospho-SMAD2, total SMAD2, and β-tubulin (loading control) by Western blot. Two representative strains are shown, demonstrating that, at 1 h of treatment, TGFβ induces phosphorylation of SMAD2 and SB-43152 completely ablates phospho-SMAD2, whereas salinomycin has no effect. B, fibroblasts were treated with TGFβ and/or salinomycin, and cells were harvested 0, 5, 10, 30, and 60 min after TGFβ treatment to analyze levels of phospho-TAK1, total TAK1, phospho-p38, and total p38 by Western blot. Salinomycin blocked phospho-TAK production 10 and 30 min after TGFβ treatment. R.E., relative expression. C, Human fibroblasts were treated as in A, except that cells were harvested at the 0, 24, 48, and 72 h time points to analyze levels of phospho-TAK1, totalTAK1, phospho-p38, total p38, phospho-SMAD2, and total SMAD2 by Western blot. The relative expression level of each phosphoprotein (normalized to β-tubulin) as determined by densitometry is shown below each lane. Salinomycin reduced TGFβ-induced phospho-Smad2 levels more than 2-fold at 48 h. Likewise, salinomycin also inhibited phospho-TAK1 levels at 24, 48, and 72 h. Salinomycin blocked p38 phosphorylation at the 24, 48, and 72 h time points, with an 80% reduction at 24 h and more than a 3-fold reduction at 72 h. Data are representative of at least three different experiments performed with different human fibroblasts strains.
