FIGURE 1.
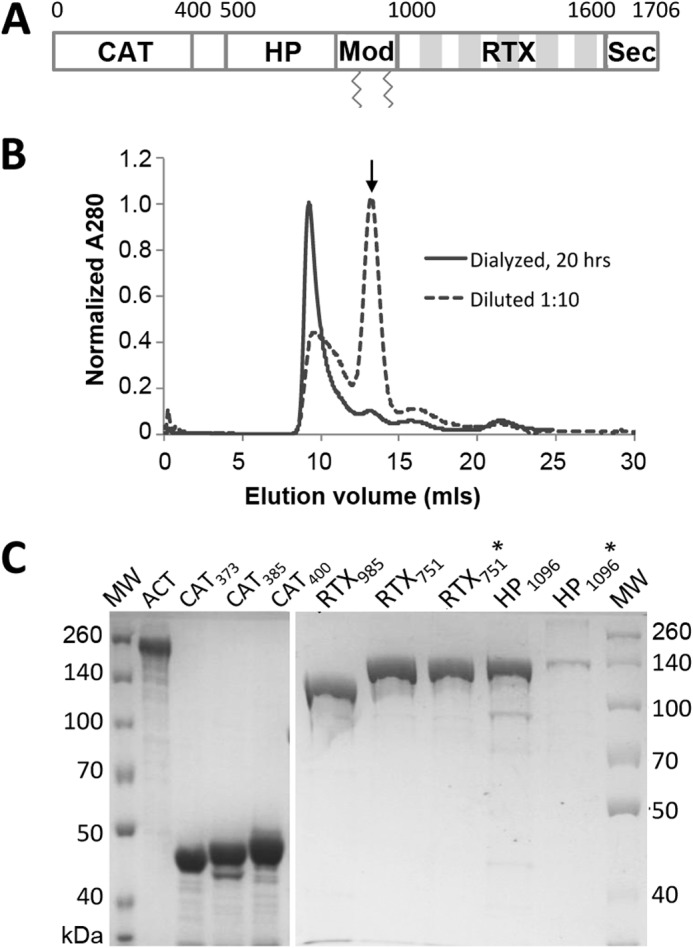
Expression and purification of intact ACT and domains. A, adenylate cyclase toxin domain architecture. ACT is a 177-kDa protein toxin, consisting of five sequential domains as follows: the catalytically active N-terminal adenylate cyclase (CAT) domain, the central hydrophobic (HP) domain, modification (Mod) region carrying two acylation sites, Lys-860 and Lys-983, the RTX domain, and finally a C-terminal secretion signal (Sec). The five shaded blocks in the RTX region represent tandem Gly-Asp-rich repeats. B, ACT forms high molecular mass species (>600 kDa) when urea is removed by dialysis or dilution. Size exclusion chromatograms of ∼120 μg of ACT using a Superdex 200 column were collected directly after a 1:10 dilution (final urea concentration 0.8 m) or overnight dialysis into HBSC with 1 m urea. Arrow indicates the size expected for monomer, 177 kDa. C, SDS-polyacrylamide gel comparing full-length ACT with different domain constructs after purification from E. coli. Three versions of the CAT domain (residues 1–373 (CAT373), 1–385 (CAT385), and 1–400 (CAT400)) and three versions of the RTX domain (residues 985–1706 (RTX985), 751–1706 (RTX751), and acylated 751–1706 (RTX751*)) were expressed from plasmid pET28a with an N-terminal His6 tag. The HP domains (residues 399–1096 (HP1096) and acylated 399–1096 (HP1096*)) were expressed from plasmid pMalc-5x with an N-terminal MBP fusion for enhanced solubility and C-terminal His6 tag.
