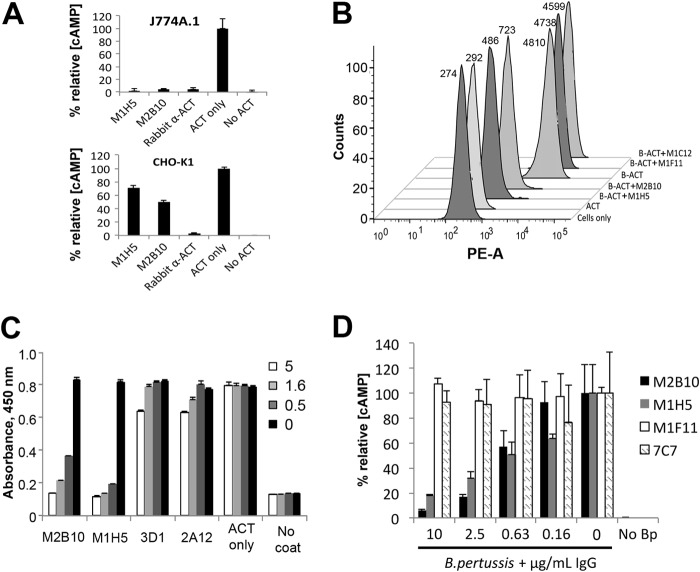
FIGURE 6.
M2B10 and M1H5 antibodies block the ACT-αMβ2 integrin interaction. A, antibody neutralization of cAMP intoxication using J774A.1 presenting the αMβ2 receptor and CHO-K1 cells lacking the receptor. Both assays were performed with a 160-fold molar excess of scAb over ACT and analyzed as in Fig. 4. B, antibody blockade of ACT binding to J774A.1 cells assessed by FACS. Biotinylated ACT was incubated with a 300-fold molar excess of scAb and added to 4 × 105 J774A.1 suspension cells on ice. After washing, bound biotinylated ACT was detected with streptavidin-PE and analyzed by FACS (mean fluorescence noted next to each peak). Controls include untreated cells (Cells only) and cells treated with nonbiotinylated ACT followed by streptavidin-PE (ACT). C, antibody blockade of ACT binding to soluble αMβ2 integrin by ELISA. ACT (0.5 μg/ml) was incubated with serial dilutions of M2B10, M1H5, 3D1 and 2A12 antibodies at 5-, 1.6-, and 0.5-fold molar excess, before transfer to an ELISA plate coated with murine αMβ2 integrin. Bound ACT was detected with rabbit anti-ACT polyclonal antibody followed by HRP-conjugated goat anti-rabbit IgG antibody. D, antibody neutralization of ACT secreted by B. pertussis. Antibodies at 10, 2.5, 0.63, and 0.16 μg/ml were incubated with live B. pertussis (A600 = 0.2) before adding to adherent J774A.1 cells. The resulting intracellular cAMP concentrations were measured, normalized to total protein concentration, and expressed as % relative cAMP.