FIGURE 4.
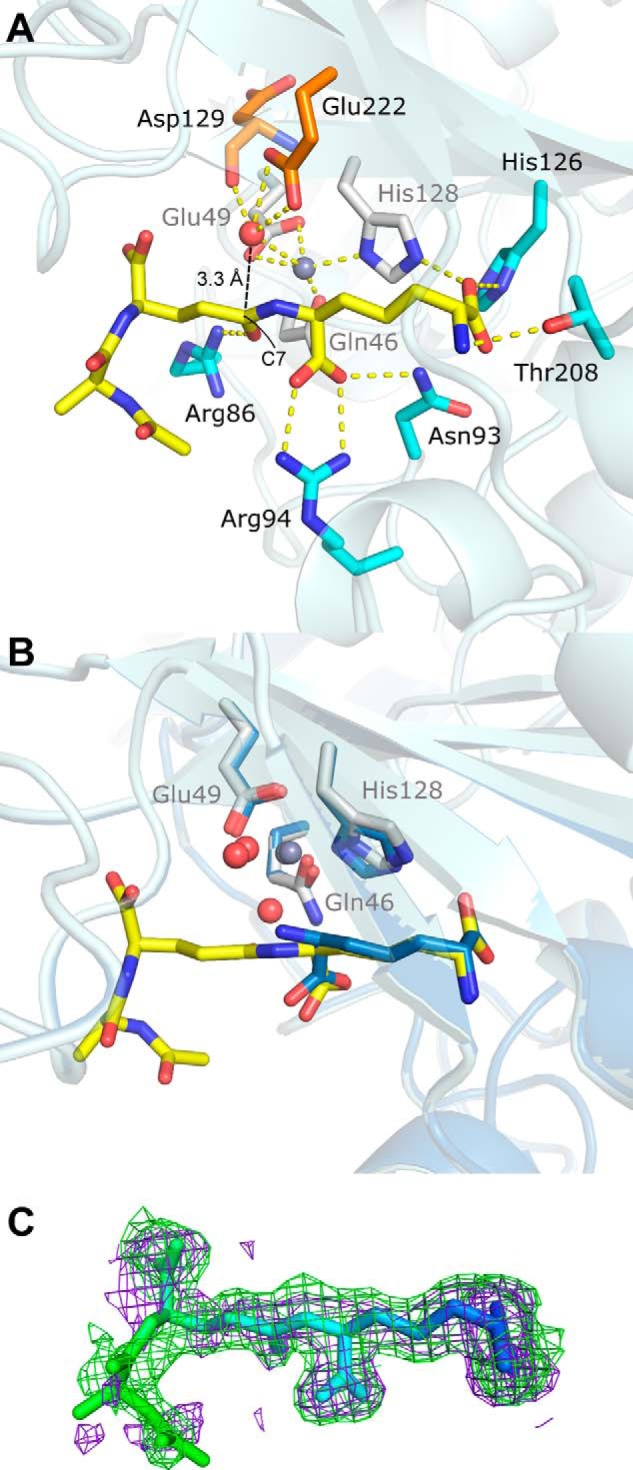
Tripeptide substrate binding site (PDB code 4WCN). A, key zinc and substrate interactions with Csd4 are shown. Zinc ligands are colored white; tripeptide ligands are colored cyan; the predicted catalytic water is red, and its ligands are orange; zinc is gray; and key interactions are shown as dotted lines. Hydrophobic residues and other waters are not shown. B, structural alignment between substrate- and product-bound Csd4. The view is of the active site in A with a 30° rotation about the x axis. C, tripeptide omit Fo − Fc difference map contoured at 2 σ generated prior to the addition of the tripeptide to the model (purple) and from the final model (green) showing well defined omit density for the deeply buried portions of the tripeptide substrate. Increased tripeptide flexibility in relation to the degree of surface exposure are indicated by a B-factor based coloring scheme (blue = ∼20 Å2 and green = ∼60 Å2).
