FIGURE 6.
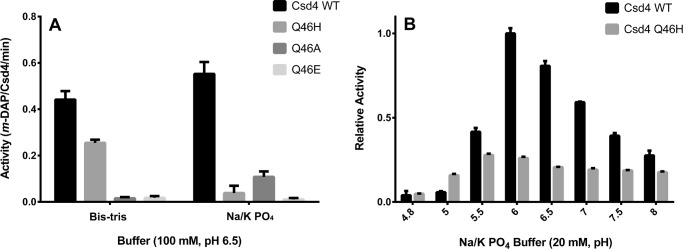
Wild-type Csd4 exhibits higher catalytic activity on the tripeptide substrate than its active site variants. A, Csd4 activity was continuously monitored via the activity of meso-diaminopimelate dehydrogenase, which consumes the Csd4 product m-DAP to produce NADPH. Buffer-based activity rate differences of Csd4 and its variants are shown. B, the pH-based activity differences between wild-type Csd4 and the Q46H variant was examined by examining the amount of product produced after 20 min. Mean values are shown with error bars representing the standard deviation based on at least three experimental replicates. The p value for all variants is <0.0005 as compared with wild type in their respective buffers utilizing the t test in A. p values between wild type and Q46H are <0.0006 for all pH values except pH 4.8 in B.
