FIGURE 2.
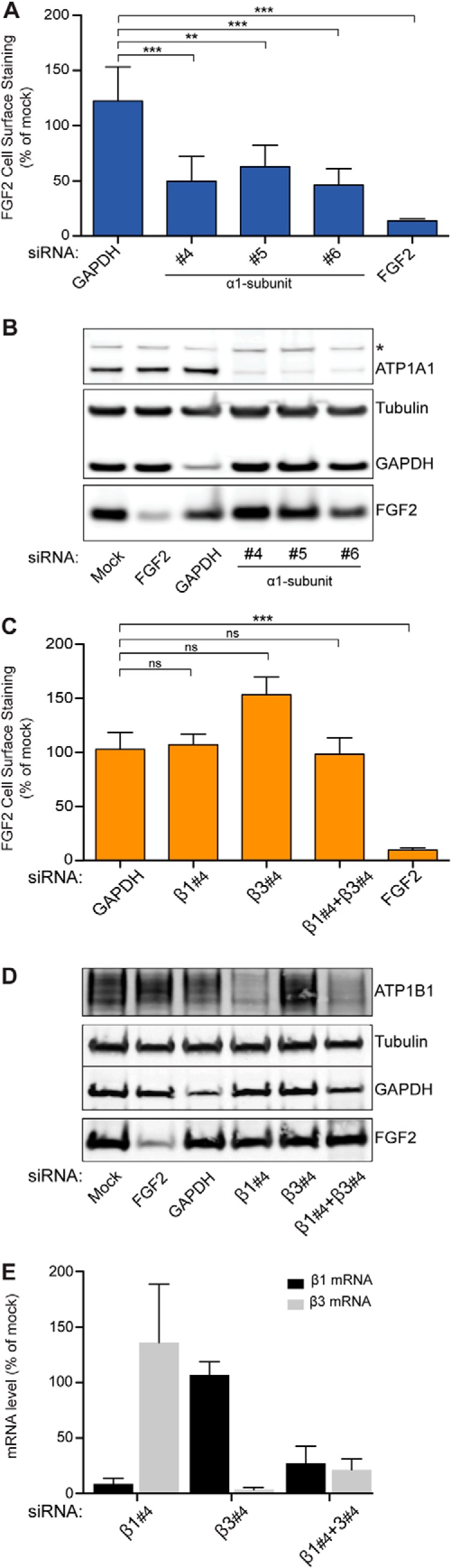
Validation of ATP1A1 as a component of the machinery mediating unconventional secretion of FGF2. A flow cytometry assay was used to quantify unconventional secretion using a stable HeLa cell line expressing FGF2 and GFP in a doxycycline-dependent manner (18, 23, 24, 30, 31). A, FGF2 cell surface expression under control conditions and after down-regulation of ATP1A1. Validated siRNAs directed against GAPDH and FGF2 were used as negative and positive controls, respectively. Error bars, S.D. (n = 4). To test whether observed differences between experimental conditions were statistically significant, an unpaired two-tailed Student's t test was performed (ns, not significant; **, p value ≤ 0.01; ***, p value ≤ 0.001). B, Western blot analysis to test the efficiency of down-regulation by RNAi for the gene products indicated. The asterisk indicates a cross-reactivity of the anti-ATP1A1 antibody used in this analysis. C, FGF2 cell surface expression under control conditions and after down-regulation of ATP1B1 and ATP1B3, the β1- and β3-chains of the Na/K-ATPase expressed in HeLa cells. Validated siRNAs directed against GAPDH and FGF2 were used as negative and positive controls, respectively. Error bars, S.D. (n = 4). To test whether observed differences between experimental conditions were statistically significant, an unpaired two-tailed t test was performed (ns, not significant; ***, p ≤ 0.001). D, Western blot analysis to test the efficiency of down-regulation by RNAi for the gene products indicated. E, quantitative RT-PCR analysis to monitor RNAi-mediated down-regulation of ATP1B1 and ATP1B3, the β1- and β3-chains of the Na/K-ATPase.
