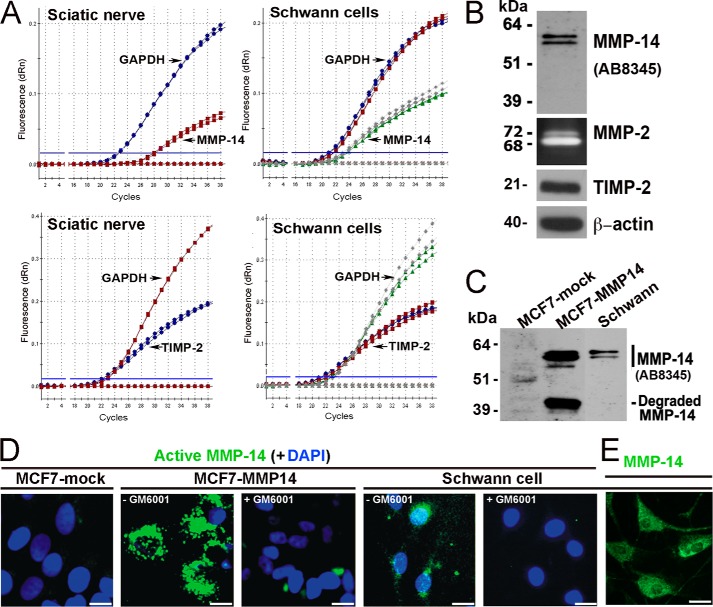
FIGURE 4.
The MMP-14/MMP-2/TIMP-2 axis in Schwann cells in vitro. A, TaqMan qPCR amplification plots for MMP-14, TIMP-2, and GAPDH (normalizer) in primary rat Schwann cell cultures (grown in DMEM containing 10% FBS for 24 h) and normal rat sciatic nerve. MMP-14 and TIMP-2 amplification closely follows GAPDH amplification (i.e. 0–5 cycle intervals between threshold cycle (Ct) values), suggesting high baseline expression of both the enzyme and its inhibitor. Data shown are from duplicate Schwann cell samples (same color curves) from two independent experiments or duplicate nerve samples (same color curves) pooled from n = 5/sample. B, immunoblotting for MMP-14 (AB8345 antibody) and TIMP-2 of Schwann whole cell lysate aliquots (5 μg/lane) and gelatin zymography of the Schwann cell medium aliquots (20 μl) for the activation status of MMP-2. β-Actin is used as a loading control. dRn, the fluorescence emission of the baseline. C, immunoblotting of MMP-14 (AB8345 antibody) in MCF7-mock, MCF7-MMP14, and Schwann cell whole cell lysate aliquots (equal amounts; 3.5 μg/lane each). D, imaging of the catalytically active cellular MMP-14. MCF7-mock, MCF7-MMP14, and Schwann cells were co-incubated for 3 h with the MP-3653 fluorescent reporter alone or jointly with the non-fluorescent hydroxamate inhibitor GM6001 (+GM6001). The resulting fluorescence of the cell-bound MP-3653 reporter recorded active MMP-14 (green). DAPI stains the nuclei (blue). Scale bars are 8 μm. E, Schwann cells immunostaining with the MMP-14 3G4 antibody are reactive with both active and inactive enzyme (green). Scale bars are 15 μm.