FIGURE 8.
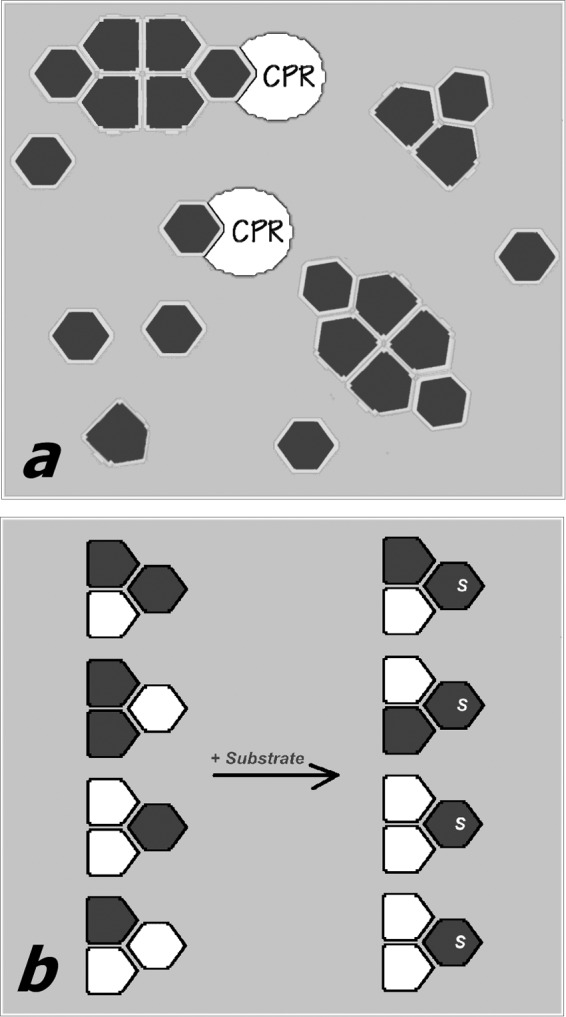
Schematic illustrating our hypothesis about the mechanism of the functional effects of P450 oligomerization. In a, a P450 heme protein (dark gray-filled polygons) exists in equilibrium between monomers and oligomers. The subunits of the oligomer attain two different conformations (hexagons and pentagons), depending on their position in the trimer, which is the minimal building block of the oligomers. One of the two conformations (hexagons) preferentially forms electron transfer complexes with the reductase (white-filled partial ovals) and interacts with the P450 substrates. The “pentagon-shaped” conformation is therefore excluded (either completely or partially) from electron transfer and catalysis. b, illustrates the hypothesis of substrate-induced reorganization of mixed oligomers of two different P450 enzymes (white- and dark gray-filled polygons). In the absence of substrate (left) the distribution of the two species between two P450 conformations (hexagons and pentagons) is random. The addition of a substrate specific to one of the two P450 species (dark gray) causes a redistribution of the two species in the oligomers, so that the gray protein now occupies the positions that are open for the interactions with substrate (hexagon-shaped subunits).
