Table 1.

| ||||
|---|---|---|---|---|
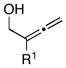
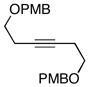
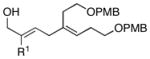
| entrya | allene | alkyne | yield (%) | 1,4-dieneb |
|
|
|
||
| 1 | 5; R1 = H | 6 | 53 | 7c; R1 = H |
| 2 | 8; R1 = Me | 74 | 9; R1 = Me | |
| 3 |
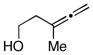 10 |
6 | 64 |
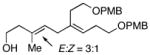 11 |
| 4 |
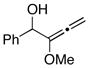 12 |
6 | 87 |
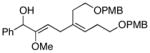 13 |
| 5 |
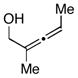 14 |
6 | 53 |
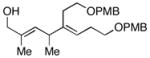 15d |
| 6 | 12 |
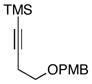 16 |
66 |
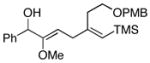 17e |
| 7 | 8 |
 18 |
48 |
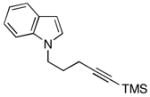
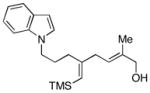 19e |
| 8 | 8 |
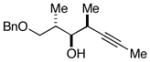 20 |
62 |
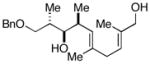 21f |
Typical reaction conditions: alkyne (1.4 eq), Ti(Oi-Pr)4 (2.1 eq), c-C5H9MgCl (4.2 eq), PhMe (−78 to −30 °C), cool to −78 °C then add allenyl alkoxide (1 eq), (−78 to 0 °C).
Products formed as single olefin isomers unless otherwise noted.
Formed as a 3:1 mixture of products containing a minor cross-conjugated triene (see Supporting Information for details).
In addition, 31% of a cross-conjugated triene was isolated from this reaction.
rs = 4:1.
rr = 2:1.
