Fig. 6. r1(En1cre)- and r4(Hoxb1cre)-derived norepinephrine neurons differ in their axon morphology at multiple target sites.
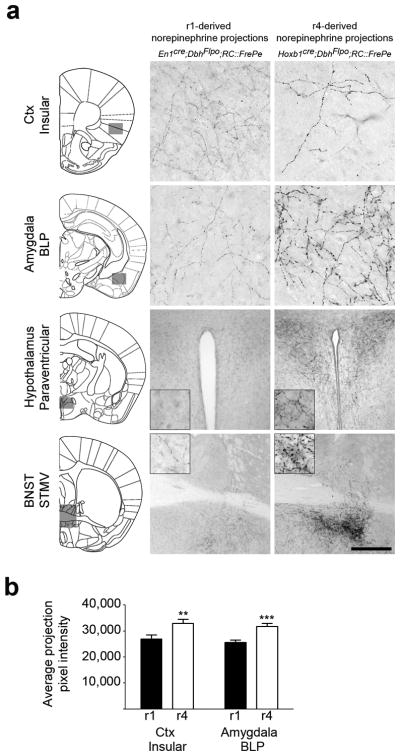
(a) Coronal sections from adult mouse brains immunostained for eGFP to detect axonal inputs from r1(En1cre;DbhFlpo;RC∷FrePe)- and r4(Hoxb1cre;DbhFlpo;RC∷FrePe)-derived norepinephrine neurons to the insular cortex, basolateral amygdala posterior part (BLP), paraventricular hypothalamus, and the bed nucleus of the stria terminalis (BNST) medial division ventral part (STMV). In the representative sections, corresponding to the boxed areas within the brain schematics (left), axons from r4(Hoxb1cre)-derived norepinephrine neurons appear thicker and have larger varicosities than those of r1(En1cre)-derived neurons. Scale bar indicates 56 μm (cortex, amygdala), 222 μm (hypothalamus), and 500 μm (BNST). (b) Quantitative comparison of projection fiber type from r1(En1cre)- and r4(Hoxb1cre)-derived norepinephrine neurons, confirms the link between genetic lineage and fiber morphology. The average projection pixel intensity of eGFP-positive axon fibers from r1(En1cre)- and r4(Hoxb1cre)-derived norepinephrine neurons projecting to the insular cortex and BLP amygdala is shown (insular cortex n=15 and BLP amygdala n=10 images from four mice; error bars are mean ± s.e.m.). A two tailed, unpaired t-test was used to determine significance **p=0.009 t=6.801 df =14 (insular Ctx), ***p=0.0006 t=2.808 df=28 (BLP amygdala).
