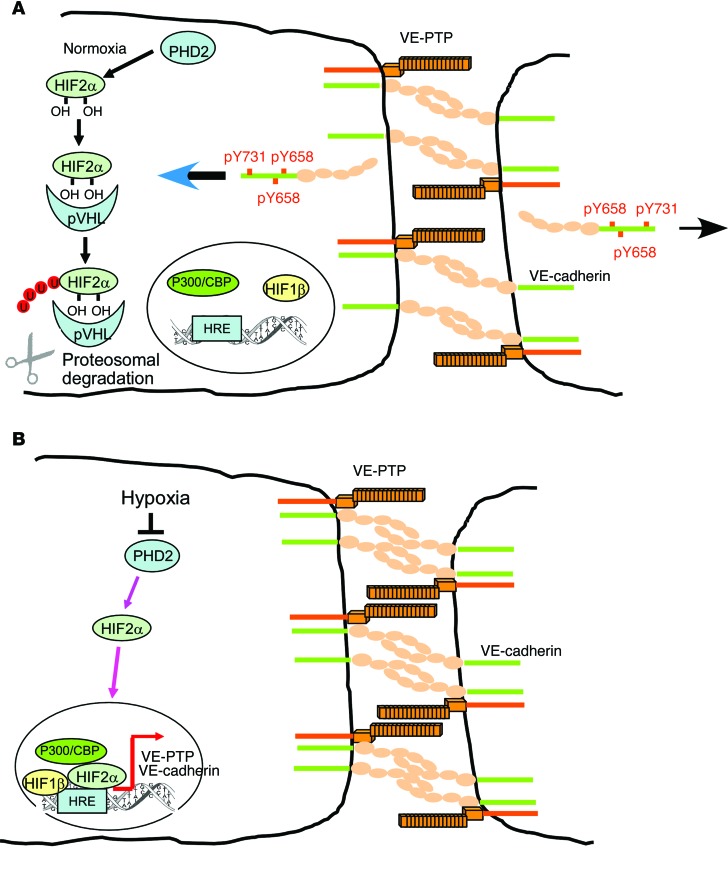
Figure 9. Signaling pathways regulating HIF2α-induced VE-PTP expression.
(A) In normoxia, PHD2 hydroxylates HIF2α, resulting in its binding to pVHL, which targets HIF2α for proteasomal degradation. Basal levels of VE-PTP and VE-cadherin are expressed in ECs to maintain a restrictive endothelial barrier. VE-PTP–induced dephosphorylation of VE-cadherin maintains VE-cadherin at AJs and prevents VE-cadherin internalization. (B) In hypoxia, PHD2 activity is inhibited, and nonhydroxylated HIF2α accumulates in the nucleus and associates with constitutively expressed HIF1β and the coactivator CBP/P300 to transactivate VEPTP gene transcription through binding to HREs. VE-PTP interaction with VE-cadherin dephosphorylates VE-cadherin at Y658, Y685, and Y731 and inhibits VE-cadherin internalization, thus enhancing AJ assembly and endothelial barrier integrity.