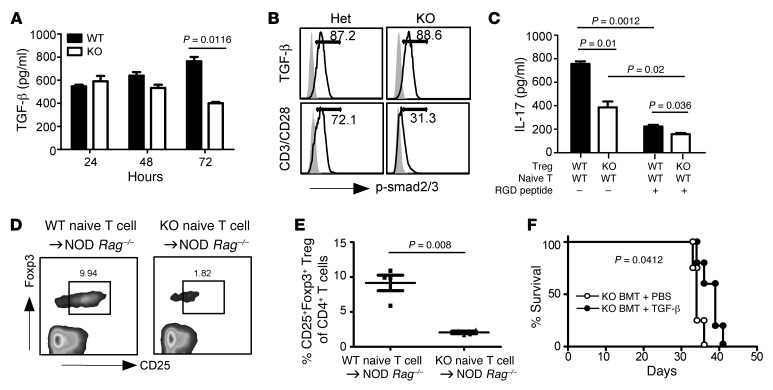
Figure 8. GP96 controls mTGF-β bioactivity.
(A) MACS-purified CD4+CD25+ Tregs were stimulated with plate-bound antibody against CD3 (1 μg/ml) and CD28 (0.5 μg/ml) for indicated times. TGF-β levels in the culture supernatant were quantitated by ELISA. Data represent 3 independent experiments. (B) CD4+CD25+ Tregs were stimulated with TGF-β1 (1 ng/ml) for 45 minutes or antibodies against CD3 and CD28 for 24 hours. Cells were then fixed and stained intracellularly for p-Smad2/3. Numbers indicate percentages of indicated cells in the entire population. Data represent 3 independent experiments. (C) CD4+CD25+ Tregs were activated with plate-bound anti-CD3 antibody (2 μg/ml) for 2 to 3 days, followed by irradiation (2000 cGy) and coculturing with CD4+CD25– naive T cells for 3 days, with or without RGD peptide. The level of IL-17A in the culture supernatant was determined by ELISA. Data represent 4 independent experiments. (D and E) 1 × 106 CD4+CD25– naive T cells from 3-week-old WT NOD or Hsp90b1fl/flCD4-Cre mice were transferred into NOD Rag–/– mice. Four weeks later, induced Tregs (CD4+CD25+FOXP3+) were examined by flow cytometry (D) and quantified (E) (each dot represents 1 individual mouse). Data represent 3 independent experiments. (F) Irradiated NOD Rag–/– recipient mice were transplanted with KO BM. Seven days later, mice were injected i.p. with recombinant TGF-β2 (0.2 μg/100 μl) daily for 5 days, followed by once every 3 days. Survival of the mice was monitored. Data represent 2 independent experiments. Statistical analyses were performed with 2-tailed Student’s t test for (A, C, and E) and log-rank test (F).