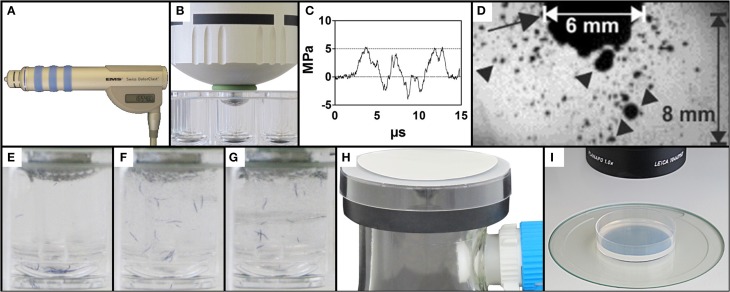
Figure 1.
Exposure of C. elegans to therapeutic shock waves. (A) Handpiece of the Swiss DolorClast therapeutic shock wave device used in the present study. (B) Placement of the shock wave device 6-mm applicator tip and specialized O-ring in a single well of a 96-well plate. (C) Pressure as a function of time of the shock waves applied in the present study. Measurements were taken at 5 mm distance to the applicator with a fiber optic probe laser hydrophone (FOPH, 2000; RP Acoustics, Leutenbach, Germany) coupled to an oscilloscope (LeCroy 9361; LeCroy, Chestnut Ridge; NY). (D) Cavitation bubbles (arrowheads) produced by the shock waves applied in the present study. The arrow points to the 6-mm applicator of the shock wave device. The size of the cavitation field generated by the shock wave device as operated in the present study guaranteed that each worm was always exposed to cavitation when subjected to a shock wave, regardless of the position of the worm within the well. The image was taken with a high-speed CCD camera (Photron Ultima APX; Photron, Tokyo, Japan) with a framing rate of 300,000 frames per second and an exposure time of 1/2700,000 s. (E–G) Mixing property of shock wave application as shown by position of blue stained worms following 0, 10, and 50 shock waves, respectively, ensuring random, uniform exposure of C. elegans worms to shock waves. (H) Rapid-transfer method using membrane filters and vacuum suction to transfer C. elegans worms from liquid media to agar-plates in a matter of seconds. (I) Position of a worm-containing agar-plate under a dissecting microscope prepared for video capture.