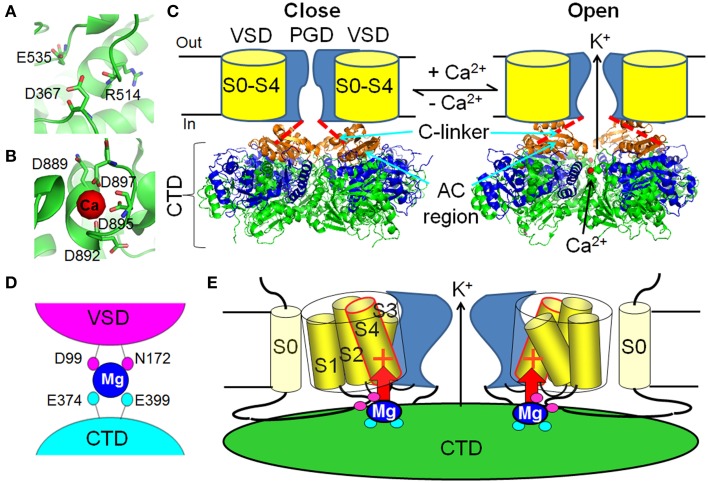
Figure 2.
Ca2+ and Mg2+-dependent activation of BK channels. (A) The putative Ca2+ binding pocket in the RCK1 site (PDB ID: 3NAF). (B) The Ca2+ binding pocket in the Ca2+ bowl site (PDB ID: 3U6N). (C) Ca2+ binding changes the conformation of the cytosolic tail domain (CTD), which pulls the C-linker to open the activation gate of BK channels. The Ca2+-free (3NAF) and Ca2+ bound to the Ca2+ bowl (3U6N) CTD structures are shown in the left and right panels, respectively. One of the most dramatic Ca2+-induced conformational changes happens in the AC region (β A–αC, orange). The rest of the RCK1 domain is shown in blue and the RCK2 domain is shown in green. The bound Ca2+ in the Ca2+ bowl is shown as red dot in the right panel. (D) The low affinity Mg2+ binding site is composed of D99 and N172 in the voltage sensory domain (VSD) and E374 and E399 in the CTD. Magenta and cyan of these residues illustrate that D99/N172 and E374/E399 are from neighboring Slo1 subunit. (E) Mg2+ binds to the interface of the VSD and the CTD to activate BK channels through electrostatic interaction with the voltage sensor. The red + sign in S4 represents the major voltage sensing residue R213.