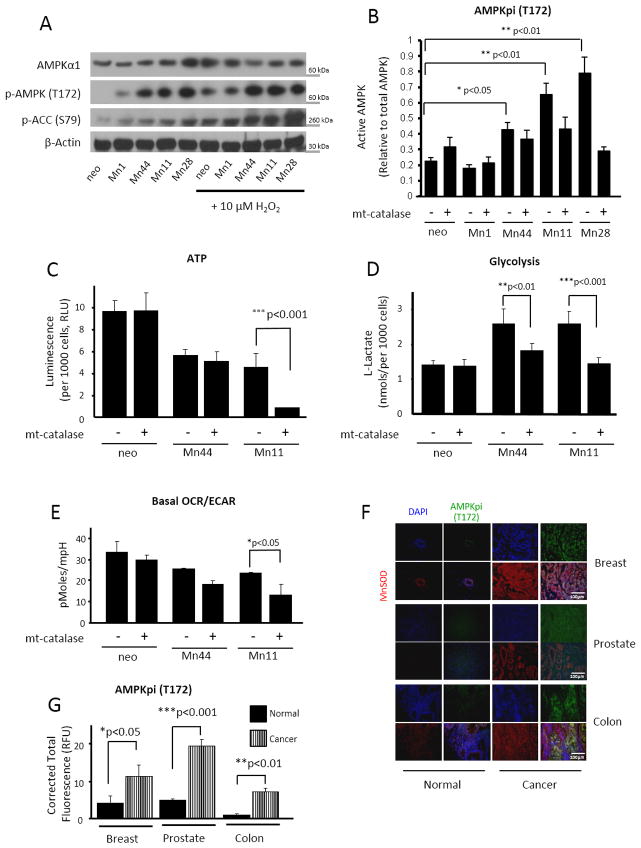
Figure 5. AMPK activation is required for the MnSOD-driven glycolytic shift.
(A) Western blot analysis of AMPK activation. Active AMPK was examined using phospho-AMPKα1 (Thr172) and phospho-ACC (Ser 79) as surrogates. Treatment of cells with exogenous H2O2 (5 μM) for 30 minutes significantly increased AMPK activation in neo, Mn1 and Mn44 but did not further enhanced AMPK activity in Mn11 and Mn28. (B) Transfection of mito-catalase into neo and MnSOD overexpressing cells reduced AMPK activation in Mn11 and Mn28 indicating that H2O2 originating from mitochondria activates AMPK. Mito-catalase gene was delivered to MCF7 cells using a lentiviral construct 72h prior to harvesting (C) Treatment with mito-catalase reduced steady state levels of ATP in Mn11 indicating that cells expressing high MnSOD levels depend on mtH2O2 and AMPK activation to support ATP production. Steady state ATP levels were assessed by a chemiluminescence employing luciferin/luciferase mixtures (D) Diminished lactate production by mito-catalase in Mn44 and Mn11 further indicated that MnSOD overexpression via mtH2O2 enhances glycolysis which in turn sustains ATP production. (E) Mito-catalase did not affect the OCR/ECAR ratio in neo cells but significantly altered OCR/ECAR in Mn44 and Mn11 indicating that that MnSOD overexpression leads to the metabolic shift to glycolysis via increasing mtH2O2 outflow. (F) Staining of phospho-AMPKα1 (Thr172) in cancerous human breast, prostate and colon tissue compared to control normal tissue. AMPK phosphorylation (green staining) paralleled an increase in MnSOD expression (red staining). Tissues were collected, processed, graded and stored by the University of Illinois tissue bank (G) Quantification of phospho-AMPKα1 (Thr172) and MnSOD fluorescence staining performed using ImageJ and statistical analysis was performed by one-way ANOVA (GraphPad InStat). *p<0.05; **p<0.01; ***p<0.001.