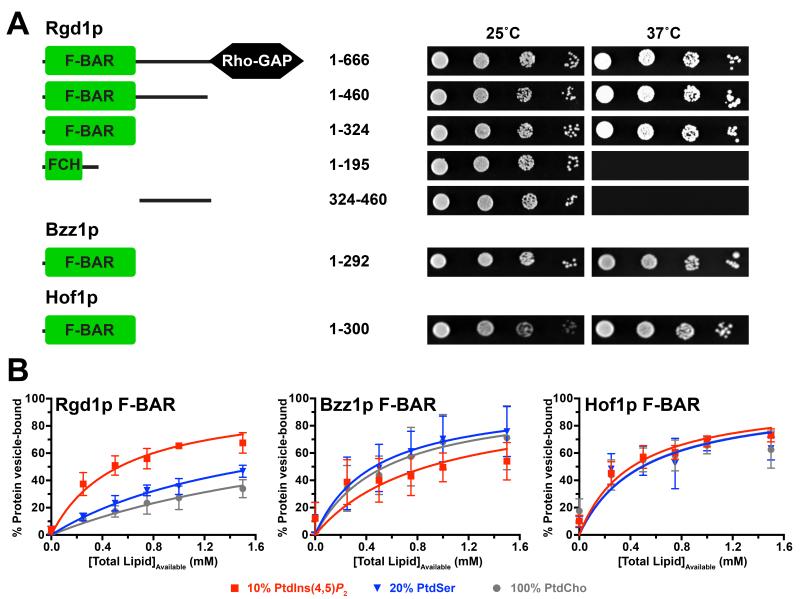
Figure 1. The Rgd1p F-BAR domain has distinct membrane binding properties.
(A) Ras Rescue (Isakoff et al., 1998) studies show that the N-terminal 324 amino acids (1-324, corresponding to the F-BAR domain) of Rgd1p are necessary and sufficient to recruit non-farnesylated Ha-RasQ61L fusions to the membrane and rescue growth of cdc25ts yeast cells at 37°C. Bzz1p and Hof1p F-BAR domains (residues 1-292 and 1-300 respectively) also show membrane recruitment. Schematic figures of the proteins fused to Q61L Ras are shown at left. On the right, representative results are shown for serial dilutions of yeast cultures expressing the noted fragments spotted in duplicate onto selection plates and incubated at the permissive (25°C) or restrictive (37°C) temperature for 4-5 days.
(B) Vesicle sedimentation studies with histidine-tagged F-BAR domains (10 μM) incubated with increasing concentrations of SUVs containing 20% (mole/mole) PtdSer or 10% (mole/mole) PtdIns(4,5)P2. The percentage of protein pelleting with the vesicles was measured, and data fit as described (Kavran et al., 1998). Mean and standard deviation (SD) are shown for at least three independent experiments.