Borderline ovarian tumors (BOTs) have been a challenge for patients, pathologists, and oncologists. For the group of patients with invasive implants, there is no consensus regarding standard therapy. This meta-analysis examines the benefits, or lack thereof, of platinum-based adjuvant treatment for BOT, showing that at present there is no evidence to support this treatment form.
Keywords: Borderline ovarian tumors, Platinum-based treatment, Chemotherapy, Implants, Adjuvant treatment
Abstract
Background.
Treatment of borderline ovarian tumors (BOTs) remains contentious, and there is no consensus regarding therapy for BOTs with invasive implants (BOTi). The benefits of platinum-based adjuvant treatment were evaluated in patients with BOTi at primary diagnosis.
Methods.
The PubMed database was systematically searched for articles using the following terms: ((borderline) OR (low malignant potential) AND (ovarian)) AND ((tumor) OR (cancer)) AND (invasive implants) AND ((follow-up) OR (survival) OR (treatment) OR (chemotherapy) OR (adjuvant treatment) OR (surgery) OR (surgical treatment)).
Results.
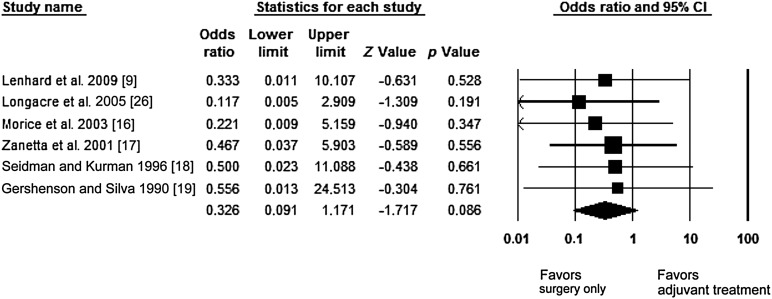
We identified 27 articles including 3,124 patients, 181 with invasive implants. All studies provided information regarding mortality or recurrence rates. Central pathological examination was performed in 19 studies. Eight studies included more than 75% stage I patients; 7 included only advanced-stage patients, and 14 included only serous BOT. The pooled recurrence estimates for both treatment groups (adjuvant treatment: 44.0%, upfront surgery: 21.3%) did not differ significantly (p = .114). A meta-analysis of the 6 studies providing separate mortality data for both treatment groups favored surgical treatment only, but this difference did not reach statistical significance (.05 < p < .1; odds ratio: 0.33; 95% confidence interval: 0.09–1.71; p = .086). We were unable to pool the results of the included studies because not all studies registered events in both treatment groups. Egger’s regression indicated low asymmetry of the studies (p = .39), and no heterogeneity was found (I2 = 0%).
Conclusion.
We did not find evidence supporting platinum-based adjuvant therapy for BOT with invasive implants.
Abstract
背景. 目前对卵巢交界性肿瘤(BOT)的治疗仍存有争议,有关 BOT 伴浸润性种植(BOTi)的治疗也未达成共识。本研究在首次诊断为 BOTi 的患者中对以铂类为基础的辅助治疗的获益进行了评价。
方法. 我们使用下列检索词在 PubMed 数据库中对文献进行了系统性检索:((交界性)或(低度恶性潜能)和(卵巢))和((肿瘤)或(癌症))和(浸润性种植)和((随访)或(生存)或(治疗)或(化疗)或(辅助治疗)或(外科)或(外科手术治疗))。
结果. 共纳入 27 篇文献,包括 3 124 例患者,其中 181 例伴有浸润性种植。所有研究都有死亡率或复发率数据。19 项研究进行了中心实验室病理检查,8 项研究纳入的 I 期患者比例> 75%,7 项研究只纳入了进展期患者,14 项研究只纳入浆液性 BOT 患者。两个治疗组的汇总复发估算值(辅助治疗:44.0%,一期手术:21.3%)无显著差异(P=0.114)。对 6 项有两个治疗组单独死亡率数据的研究进行了 meta 分析,结果显示有利于仅手术治疗,但差异无统计学意义(0.05 < P < 0.1,比值比:0.33;95%可信区间:0.09 ∼ 1.71;P = 0.086)。由于并非所有的研究都记录了两个治疗组的事件,因此无法汇总所有纳入研究的结果。Egger 回归分析显示了低不对称性(P = 0.39),但无异质性(I2 = 0%)。
结论. 我们没有发现支持以铂类为基础的辅助治疗用于BOT伴浸润性种植的证据。The Oncologist 2015;20:151–158
Implications for Practice:
Borderline ovarian tumors (BOTs) have been a challenge for patients, pathologists, and oncologists. For the group of patients with invasive implants, there is no consensus regarding standard therapy. Platinum-based regimens are recognized as the standard chemotherapy for ovarian cancer, achieving the highest response rates and the longest survival; however, this outcome does not seem to be the case in the setting of BOT with invasive implants. This meta-analysis examines the benefits, or lack thereof, of platinum-based adjuvant treatment for BOTs.
Introduction
Borderline ovarian tumors (BOTs) were first described by Taylor in 1929 [1] and have been a challenge for both pathologists and oncologists. BOT is a disease of younger, fertile women, generally with a benign course; however, a minority of patients progress and eventually succumb to the disease. Although the corrected survival for patients with disease confined to the ovary is 100% at 15 years [2], >30% of patients with serous BOT with invasive implants will develop persistent or recurrent tumor, most commonly low-grade serous carcinoma [3]. For the group of patients with invasive implants, there is no consensus regarding standard therapy. Some oncologists would agree that adjuvant treatment is warranted for these patients, based on their worse prognosis and the tendency of these tumors to recur, not only as BOT but also as a low-grade ovarian neoplasm; however, the response of such tumors to systemic chemotherapy needs to be clarified. Platinum-based regimens are recognized as the standard chemotherapy for ovarian cancer, achieving the highest response rates and the longest survival.
At present, chemotherapy is offered mostly to patients with invasive implants, regardless of histological subtype. We aimed to clarify the effect of platinum-based adjuvant treatment on women of any age with a histological diagnosis of BOT of any variant compared with no adjuvant treatment and with regard to progression-free and overall survival and response rates to adjuvant treatment.
Methods
Articles selected for inclusion were English-language, peer-reviewed articles that examined the effect of platinum-based chemotherapy on the treatment of BOT of any variant presenting with invasive implants at primary diagnosis (BOTi). We considered all articles, regardless of whether the studies were retrospective or prospective.
We excluded reports that did not state outcomes from BOTi patients separately from outcomes of BOT patients with noninvasive implants or without implants. Also excluded were studies that did not report the follow-up of patients who received adjuvant treatment separately from those receiving other treatments and those that did not use a platinum-based regimen (at least in a group of patients); reports treating patients with surgery only were considered. Reports that stated outcomes from ovarian cancer patients reported together with BOTi were also excluded, as were case reports, which represent unusual occurrences and/or disease courses.
Included in the analysis were women of any age with a histological diagnosis of primary BOT of any variant with the presence of invasive implants at primary diagnosis. Women who did not have an up-to-date histological diagnosis of BOTi (according to Bell et al. [4] and women with concurrent ovarian cancer or other malignant tumors of the ovary were excluded. We further excluded any form of recurrent BOT.
We considered articles in which at least one group of patients with BOTi received adjuvant chemotherapy with platinum-based regimens and articles in which at least one group of patients with BOTi were treated by upfront surgery only.
The primary and secondary endpoints were overall survival and disease-free survival, respectively.
Search Strategy and Identification and Selection of Studies
The PubMed database was searched using the following search terms: ((borderline) OR (low malignant potential) AND (ovarian)) AND ((tumor) OR (cancer)) AND (invasive implants) AND ((follow-up) OR (survival) OR (treatment) OR (chemotherapy) OR (adjuvant treatment) OR (surgery) OR (surgical treatment)). All titles and abstracts retrieved by electronic searches were examined. The full text of potentially relevant references was obtained.
Each potentially relevant study was tabulated for each characteristic: author, title, year of publication; inclusion and exclusion criteria; study design; study population (total number of enrolled patients; total number of patients receiving adjuvant chemotherapy, radiotherapy or combination therapy; patients’ ages; histological subtype; International Federation of Gynecology and Obstetrics stage; presence and type of implants; central pathological examination); surgery details (type of initial surgery, presence of residual disease); chemotherapy details (regimen, dose and number of cycles; use for primary or recurrent disease; duration of follow-up); and outcome (recurrences, progression to carcinoma or death; patient status [dead of disease, dead of other causes, alive with disease, or alive with no evidence of disease]; type of response to treatment).
Odds ratio (OR) of mortality reduction is the primary measure of treatment effect. The OR was imputed using the raw data provided in the studies. OR and 95% confidence intervals (CIs) for each side effect were calculated.
Pooled recurrence and mortality estimates were computed using random-effects models. Odds ratios of mortality and recurrence reduction were computed using random-effects models to analyze the data. Egger’s regression intercept test across the different interventions was calculated using the Comprehensive Meta Analysis V2 software (Biostat, Englewood, NJ, http://www.meta-analysis.com).
The risk of bias was analyzed by funnel plot of the effect by the inverse of its standard error. The symmetry of such funnel plots was assessed formally with Egger's test. The heterogeneity was assessed formally by calculating I2.
Results
Study Selection
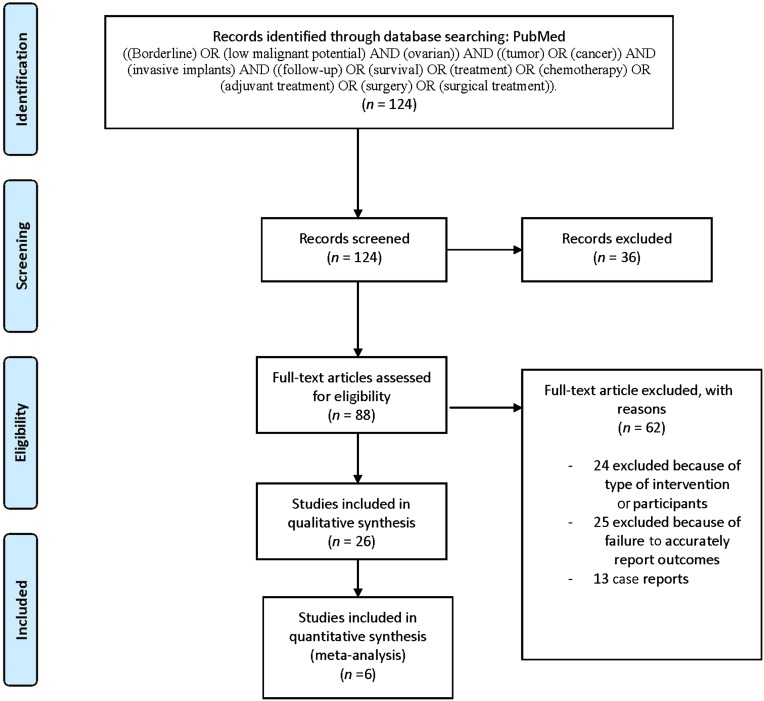
We identified 124 studies fulfilling our electronic search criteria regarding adjuvant treatment. After screening by title and abstract, 88 full-text articles were assessed. Of those, 25 were excluded because the follow-up of patients with BOTi was presented together with other BOT patients or the follow-up of patients who underwent chemotherapy for BOTi was presented together with those who underwent chemotherapy for BOT. Another 7 articles were excluded because they did not include BOTi, and 12 articles were excluded because chemotherapy was either entirely non-platin-based or entirely nonspecified. We excluded 2 articles that included only patients with recurrent BOT, 13 case reports, and 3 articles that also included patients with invasive ovarian cancer (IOC). A total of 26 studies were available for final analysis (Fig. 1).
Figure 1.
PRISMA flow diagram.
Study Characteristics
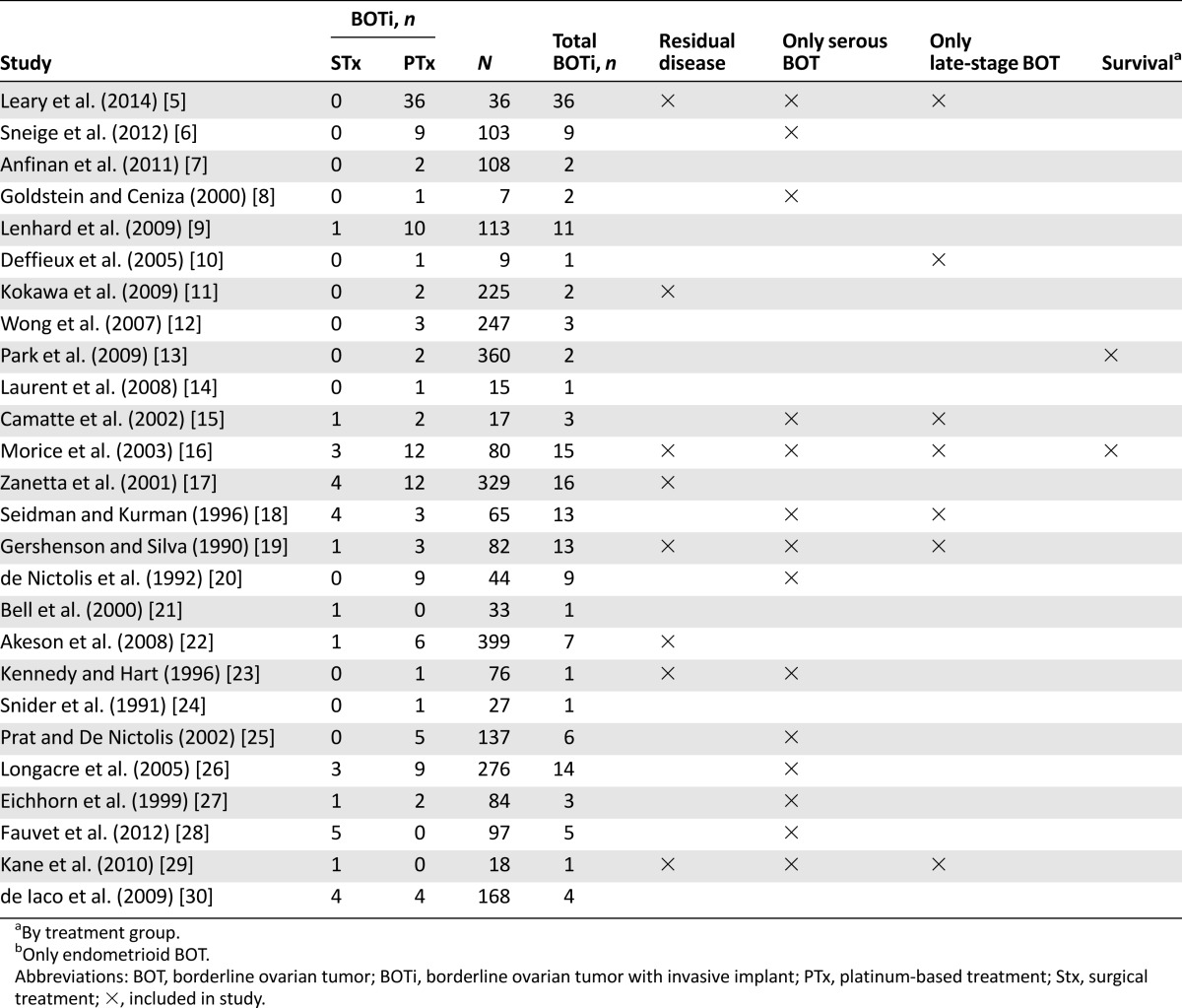
According to the inclusion criteria, all studies considered at least one patient with histologically confirmed primary BOT with invasive implants. The characteristics of the reviewed studies and topics addressed by the authors are shown in Table 1. All studies were retrospective in nature. Together 3,124 patients were pooled, including 181 patients with BOTi. There was a mean of 120 patients with BOT per study, a mean of 7 BOTi patients receiving platinum-based adjuvant treatment per study, and a mean of 1 BOTi patient per study being treated by surgery only. All studies reported the duration of follow-up. The mean follow-up ranged between 16 and 122 months.
Table 1.
Characteristics of the pooled studies
Participant Characteristics
The mean age of the patients ranged from 21 to 55 years. In 8 studies, >75% of patients were stage I, whereas 7 studies included only advanced-stage patients.
Overall, 18 studies carried out central pathological examinations. The studies included 2,019 patients with serous BOT, 1,014 patients with mucinous BOT, 50 patients with endometrioid BOT, and 48 with other types of BOT (mixed and clear cell tumors). Of these studies, 14 included only patients with serous BOT and 1 study included only patients with endometrioid BOT. Regarding the presence of implants, 181 patients had invasive implants, of which 132 underwent platinum-based treatment, 30 underwent surgical treatment only, and the remaining patients underwent other forms of adjuvant treatment (Table 1).
Interventions: Upfront Surgical Treatment, Adjuvant Treatment
Upfront Surgical Treatment
In some of the studies, it was not possible to ascertain the completeness of staging because it was not mentioned. Whenever the authors did not mention how many patients were staged but rather stated how many patients underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, unilateral salpingo-oophorectomy, or omentectomy separately, the staging rate was calculated based on the number of patients treated with at least O, which denotes intent to stage. According to this method, the following rates were achieved: Camatte et al., 100% [15]; Morice et al., 88% [16]; Seidman and Kurman, 78% [18]; Laurent et al., 53% [14]; Gershenson and Silva, 49% [19]; Park et al., 41% [13]; de Nictolis et al., 30% [20]. The following studies specifically mentioned the rate of comprehensive or complete staging (inspection of all peritoneal surfaces, peritoneal washings, omentectomy, and multiple biopsies): 100% by Akeson et al. [22], Leary et al. [5], and Anfinan [7]; Snider et al., 85% [24]; Lenhard et al., 76% [9]; Wong et al., 66% [12]; De Iaco et al., 53% [30]; Zanetta et al., 44% [17]; Fauvet et al. [28] and Longacre et al. [26], both 29%. The study by Kane et al. did not specify the comprehensiveness of staging but included only advanced stage, denoting some degree of staging [29]. Eichhorn et al. [27], Goldstein and Ceniza [8], Kennedy and Hart [23], Bell et al. [21, 31], and Deffieux et al. [10] mentioned the number of staged patients but provided no definition of staging, with 100%, 100%, 98%, 48%, and 23%, respectively. Sneige reported 100% staging through peritoneal biopsies and washings and at least omentectomy [6]. Prat and De Nictolis [25] did not mention the extent of surgical staging; however, 37.7% of patients in the study had advanced stage disease.
Eight studies reported the definition and/or number of patients undergoing complete cytoreduction. The following studies classified optimal cytoreduction as no visible evidence of disease, and it was achieved in the following proportions of cases: Akeson et al., 99% [22]; Kokawa et al., 98% [11]; Kennedy and Hart, 98% [23]; Zanetta et al., 91% [17]; Leary et al., 86% [5]; Kane et al., 83% [29]; Gershenson and Silva, 48% [19]; Morice et al., 28% [16].
Of the studies that reported the rates of optimal cytoreduction, one emphasized the relationship between complete cytoreduction and improved outcomes. Kokawa et al. reported that the absence of residual tumors was significantly associated with improved survival rates, although the p value was not given [11]. Two studies did not find a significant relationship between residual tumor characteristics and outcomes during the follow-up period [16, 19].
Adjuvant Treatment
None of the studies provided information about the dosage and schedules of treatment, providing only the information “platin-based” treatment. We included any study with patients with BOTi in which at least a group underwent platin-based treatment as long as the follow-up of these patients was independently discernable in the article. Because of the low number of patients, we did not set a minimum number of patients per study.
Outcomes
Survival Analysis
Only one of the studies specifically analyzed the impact of chemotherapy on patients with invasive implants. The only study analyzing this specific issue had no control group. It included 36 patients with serous BOTi treated with surgery and platinum-based adjuvant treatment, 13 of which relapsed at a median of 27.3 months, 8 (22%) with invasive disease. Despite the absence of a control group treated only with upfront surgery, the authors suggested a possible role for adjuvant chemotherapy in BOTs with invasive implants.
Recurrences and Mortality
The studies reported recurrence and mortality rates according to different categories of study (e.g., staging, surgery, or individual description of the patients who suffered a recurrence). These numbers were used to calculate the number of events in the adjuvant treatment group and in the surgery group. For studies that provided the details of patients who recurred and died in the text, these details were taken into account in the calculations, considering only patients treated with platinum-based regimens (Tables 2, 3). The pooled recurrence estimate was 44.0% (95% CI: 0.354–0.529) for patients with BOTi (n = 162) undergoing adjuvant treatment and 21.3% (95% CI: 0.087–0.435) for patients undergoing upfront surgical treatment. The recurrence reduction was not statistically significant (p = .114). Considering only the studies including serous BOTi (n = 102), the recurrence reduction remains not significant (p = .181); the pooled recurrence estimate was 44.3% (95% CI: 0.349–0.551) for patients undergoing adjuvant treatment and 23.1% (95% CI: 0.077–0.521) for those undergoing surgical treatment.
Table 2.
Recurrence and mortality data from the pooled studies
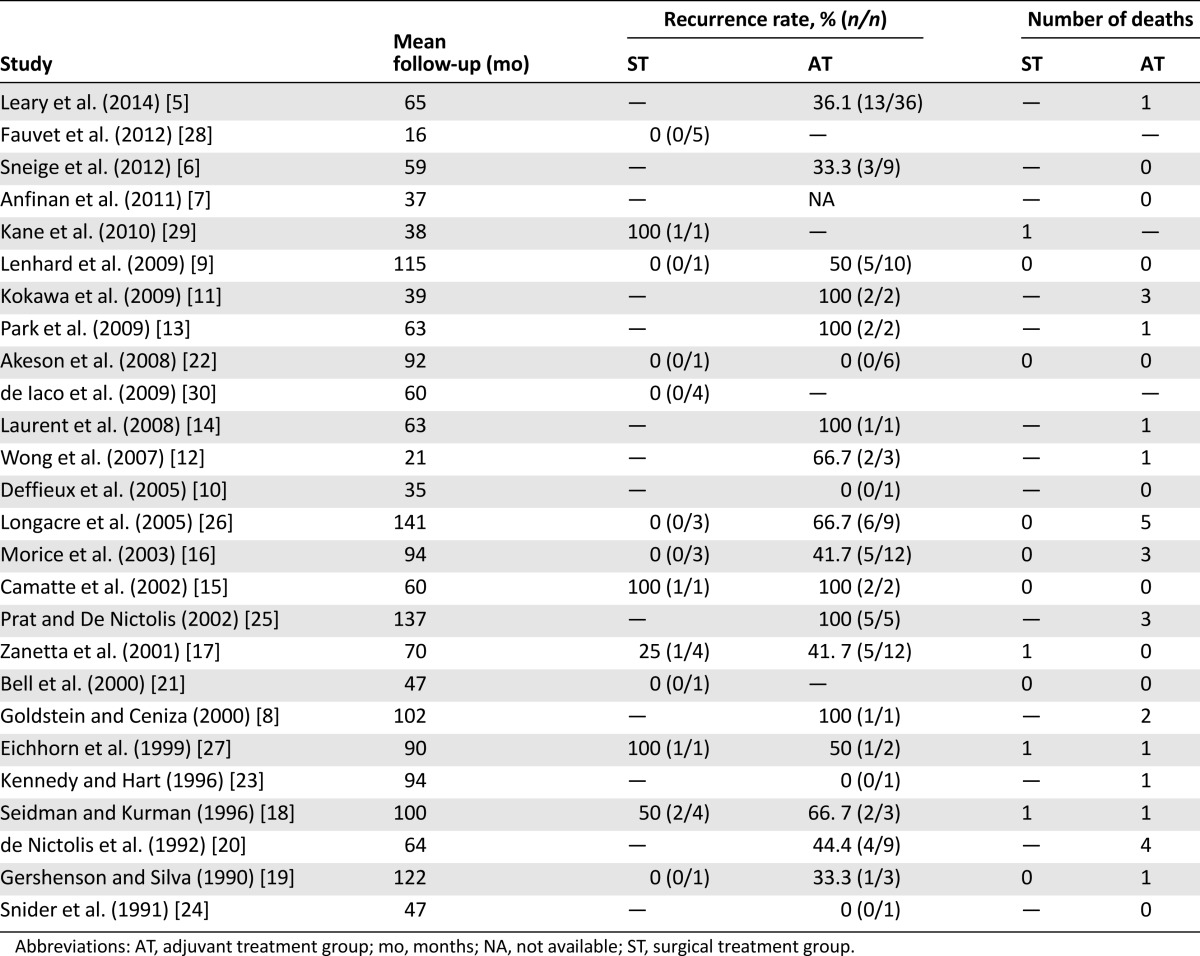
Table 3.
Mortality summary data for each intervention group in the pooled studies
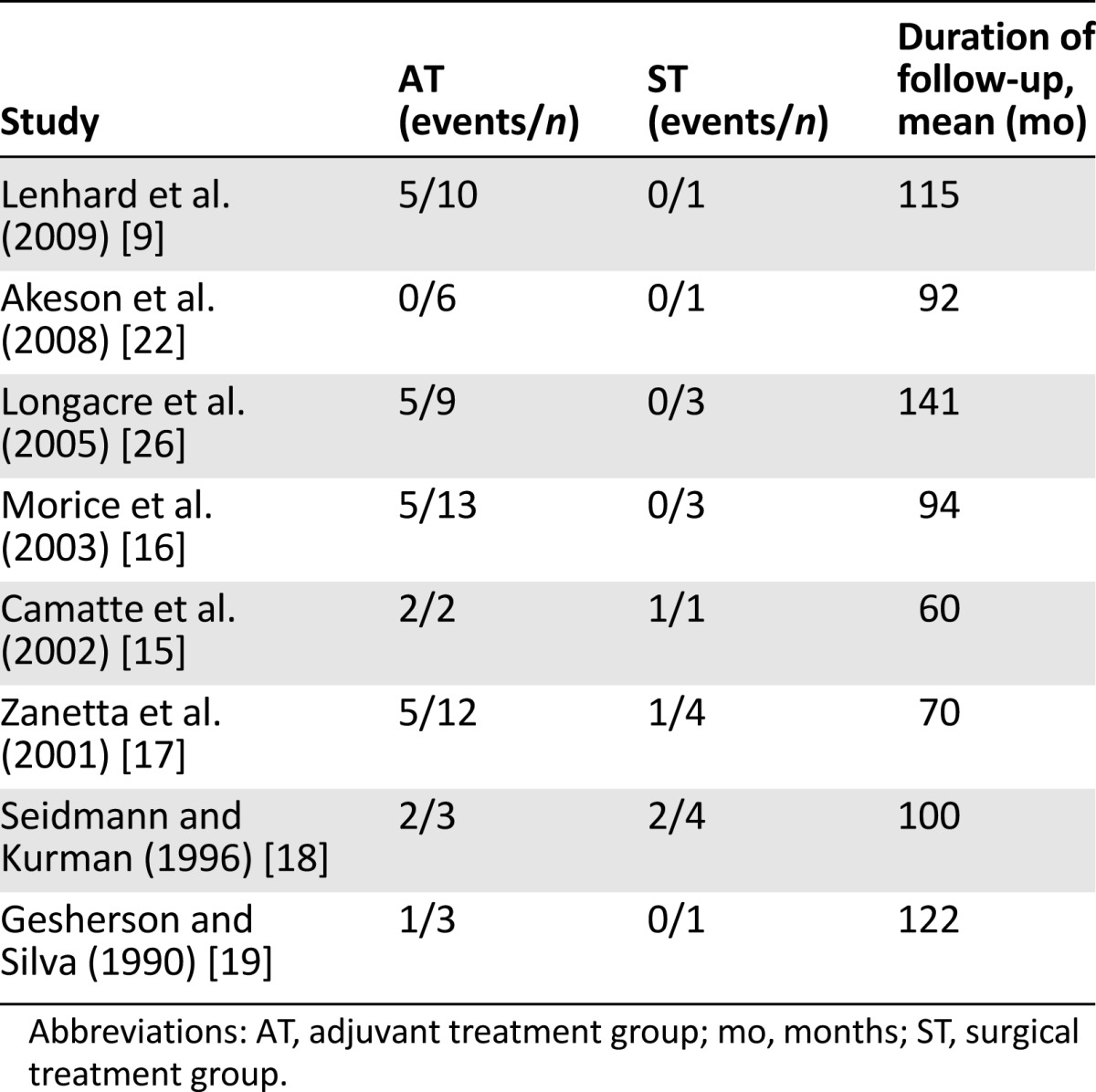
The OR of mortality reduction with 95% confidence intervals was the primary measure of treatment effect and was calculated for each side effect. The meta-analysis of the studies providing the numbers of events separately for the adjuvant treatment group and the surgery-only group was performed. Separate mortality data for both treatment groups was available for 8 studies, 2 of which were excluded because no deaths were reported (Table 3). Consequently, our estimate is based on six studies. In the pooled analysis, upfront surgical treatment showed only a trend toward lower mortality, but this difference did not reach statistical significance (.05 < p < .1; OR: 0.33; 95% CI: 0.091–1.171; p = .086) (Fig. 2). There was low evidence of asymmetry, indicating low risk of bias, as shown by Egger’s test (p = .39). No evidence of heterogeneity was shown (I2 = 0%).
Figure 2.
Forest plot showing the odds ratio, confidence interval, Z value, and p value for each side effect (surgery and adjuvant treatment) and pooled odds ratio of mortality reduction with 95% CIs.
Abbreviation: CI, confidence interval.
Discussion
This paper is the first review of exclusively platinum-based regimens in the treatment of primary BOT presenting with invasive implants at primary diagnosis. Peritoneal implants are found at initial diagnosis in 20%–46% of patients with serous BOT [23], of which 83%–96% are noninvasive [32]. Given the low prevalence of invasive implants in a disease that is uncommon, this study benefits from the inclusion of 3,124 patients, 181 with invasive implants, representing every age group, with a median age range from 21 to 55 years. The review further benefits from the inclusion of 18 studies that performed a central pathological evaluation and from even distribution regarding disease stage; 7 studies included only the more unusual advanced-stage patients, and 8 studies included >75% stage I patients. We opted to include only platinum-based regimens because current consensus statements indicate that standard chemotherapy for ovarian cancer should include a platinum-based compound [33–35]. Currently, all histological subtypes of epithelial ovarian tumors are treated with the same first-line adjuvant treatment, although serous, mucinous, and endometrioid tumors are thought to have different ethiopathogeneses. Consequently, we opted not to select one histological subtype, although 14 studies consider only serous BOT, including 1,089 patients (102 BOTi). We encountered several difficulties related to heterogeneous patient groups, incomplete descriptions of interventions, and a general lack of information about dosage and number of treatment cycles.
Surgery forms the basis of BOT treatment; however, only 30 patients with BOTi were treated exclusively by surgery, and only 3 studies treated BOTi solely with surgery [28–30]. Evidence suggests that aggressive initial surgical debulking is critical to outcome in ovarian cancer [36, 37]. The accuracy of staging is generally reported incompletely. The calculated figures range between 29% and 100% for complete or comprehensive staging based on the surgical data provided in the different studies. Although accurate staging is crucial for the analysis of patient cohorts, not only in terms of effects of treatment but also in terms of prognostic factors, it seems that the recurrence and mortality rates do not differ substantially between these works. This question was investigated by Fauvet et al. [38], Snider et al. [24] and du Bois et al. [39] analyzing the role of restaging surgery in BOT. An upstaging rate of 14.8% was reported, but there was no difference in recurrence rates [38]. In contrast, the study by du Bois et al. reported staging quality as a prognostic factor [39]. Incomplete staging showed an elevated risk for recurrence compared with comprehensive staging (hazard ratio [HR]: 1.77; 95% CI: 1.15–2.71; p = .0091) and restaging after initial surgery had a beneficial impact with respect to progress-free survival (HR: 0.577; 95% CI: 0.36 –0.92; p = .0214) [39]. Snider et al. [24] conclude that the low yield of staging in patients with mucinous tumors (0%) does not warrant a second operation. The higher yield of staging in patients with serous tumors (30.8%) suggests that the likelihood of upstaging the disease exceeds the potential morbidity, and the procedure may be warranted [24].
The pooled recurrence estimate was 44.0% for patients with BOTi undergoing adjuvant treatment and 21.3% for patients undergoing upfront surgical treatment. Considering only the studies including serous BOTi, the pooled recurrence estimate was 44.3% for patients undergoing adjuvant treatment and 23.1% for those undergoing surgical treatment. In both cases, the recurrence reduction was not statistically significant (p = .114 and p = .181). Adjuvant chemotherapy is often reserved for patients with invasive implants or bulky unresectable residual tumors; therefore, a bias in the selection of patients with higher risk of relapse could explain the worse outcome observed in these studies for those patients receiving chemotherapy. Nonetheless, according to these data, there is no benefit in adding adjuvant treatment to upfront surgery in patients with BOTi presenting with primary disease.
Of the studies analyzing the effect of adjuvant treatment on a large cohort of patients with invasive implants, Bell et al. [31], Gershenson et al. [3], and Leary et al. [5] provide the largest published cohorts. Bell et al. included a large cohort of patients with invasive implants, followed for a median of 53 months [31]. The study included 31 patients with invasive implants, 48.4% of whom received platinum-based treatment; of those, 26.7% died, whereas none of the patients who did not receive chemotherapy died [31]. In a seminal work by Gershenson et al. including 39 patients with BOTi followed for 111 months (making it the largest published series including solely BOTi patients), 79.4% were treated with platinum-based chemotherapy [3]. The results indicated no difference between those who received platinum-based chemotherapy and those who did not. In fact, patients who received postoperative platinum-based chemotherapy had significantly worse progression-free survival than all other patients. Moreover, >30% of the patients developed persistent or recurrent tumor, most commonly serous carcinoma [3]. We were not able to include this study in our review because the raw data (i.e., separate outcome for patients who underwent chemotherapy and surgery) were not provided.
The recent work by Leary et al. included 36 patients with serous BOTi treated with surgery and platinum-based adjuvant treatment, 13 (36.1%) of which relapsed at a median of 27.3 months, 8 (22%) with invasive disease [5]. Their cohort compares favorably with the rate of relapse in the form of carcinoma (20%–30%) described in the literature for BOTs with invasive implants, and the authors concluded that chemotherapy plays a favorable role in BOTi [5]. Because the findings of this study are inferential as a result of the methodology used, the robustness of the conclusions maybe overstated, requiring caution in interpretation. As shown by the present review, most BOTi patients (72.9%) reported in the literature are treated with chemotherapy, with a pooled recurrence rate of 44.3%. The recurrence estimate for invasive disease was impossible to pool because although the number of patients that recurred as invasive ovarian cancer is usually provided, it is not always linked to BOTi cases. Although the pooled recurrence estimate for adjuvant treatment was higher than that reported by Leary et al. [5], the pooled recurrence estimate for patients undergoing surgical treatment only was lower than that reported by Leary et al., at 23.1%. The retrospective study by Leary et al. permits only comparative analysis between published treatments, reflecting a common limitation throughout the literature.
The meta-analysis resulting from pooling the results from 6 studies providing mortality data on both treatment groups showed a trend favoring surgical treatment only but did not reach statistical significance (OR: 0.33; 95% CI: 0.091–1.171; p = .086), showing low asymmetry of the studies (Egger’s test, p = .39). At present, no evidence supports the use of adjuvant treatment in patients with BOTi in the primary treatment setting, although the majority of BOTi patients are offered this treatment. Nonetheless, ∼2.3% of patients with BOT will develop invasive relapses, mostly as low-grade carcinoma (50%–75%) [3, 39]. These are known to have low responsiveness to adjuvant treatment. Of the total patients diagnosed with BOT, only ∼0.8% will suffer a high-grade invasive relapse [39]; therefore, it is unlikely that it will be possible to demonstrate the global efficacy of adjuvant treatment in these patients. In the recurrent setting, it is possible that patients recurring as high-grade ovarian carcinoma will profit from adjuvant treatment.
The most common problem encountered in studies is the broad inclusion criteria, usually including women with serous and mucinous BOT and women with implants or other high-risk features. These contribute to making study interpretation changeling. It is important that future BOT studies are prospective, are well designed, and include a large cohort followed over a broad time period, even if this makes the use of international multicenter cohorts necessary. Retrospective cohorts with broad inclusion criteria (e.g., “patients with BOT”) are unlike to yield new knowledge of this disease. In the meantime, the use of first-line chemotherapy in patients presenting with primary BOTi should be discussed individually with the patient, and the patient should be informed about the lack of evidence supporting this form of treatment.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
For Further Reading: Daniela Fischerova, Michal Zikan, Pavel Dundr et al. Diagnosis, Treatment, and Follow-Up of Borderline Ovarian Tumors The Oncologist 2012;17:1515–1533.
Abstract:
Borderline ovarian tumors represent a heterogeneous group of noninvasive tumors of uncertain malignant potential with characteristic histology. They occur in younger women, are present at an early stage, and have a favorable prognosis, but symptomatic recurrence and death may be found as long as 20 years after therapy in some patients. The molecular changes in borderline ovarian tumors indicate linkage of this disease to type I ovarian tumors (low-grade ovarian carcinomas). The pathological stage of disease and subclassification of extraovarian disease into invasive and noninvasive implants, together with the presence of postoperative macroscopic residual disease, appear to be the major predictor of recurrence and survival. However, it should be emphasized that the most important negative prognostic factor for recurrence is just the use of conservative surgery, but without any impact on patient survival because most recurrent diseases are of the borderline type—easily curable and with an excellent prognosis. Borderline tumors are difficult masses to correctly preoperatively diagnose using imaging methods because their macroscopic features may overlap with invasive and benign ovarian tumors. Over the past several decades, surgical therapy has shifted from a radical approach to more conservative treatment; however, oncologic safety must always be balanced. Follow-up is essential using routine ultrasound imaging, with special attention paid to the remaining ovary in conservatively treated patients. Current literature on this topic leads to a number of controversies that will be discussed thoroughly in this article, with the aim to provide recommendations for the clinical management of these patients.
Author Contributions
Conception/Design: Ines Vasconcelos, Jalid Sehouli
Provision of study material or patients: Jessica Olschewski
Collection and/or assembly of data: Ines Vasconcelos, Jessica Olschewski
Data analysis and interpretation: Ines Vasconcelos
Manuscript writing: Ines Vasconcelos
Final approval of manuscript: Ioana Braicu, Jalid Sehouli
Disclosures
The authors indicated no financial relationships.
References
- 1.Taylor HC. Malignant and semi-malignant tumors of the ovary. Surg Gynecol Obstet. 1929;48:204–230. [Google Scholar]
- 2.Kaern J, Tropé CG, Abeler VM. A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer. 1993;71:1810–1820. doi: 10.1002/1097-0142(19930301)71:5<1810::aid-cncr2820710516>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Gershenson DM, Silva EG, Levy L, et al. Ovarian serous borderline tumors with invasive peritoneal implants. Cancer. 1998;82:1096–1103. [PubMed] [Google Scholar]
- 4.Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors: Histologic features and prognosis. Cancer. 1988;62:2212–2222. doi: 10.1002/1097-0142(19881115)62:10<2212::aid-cncr2820621024>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Leary A, Petrella MC, Pautier P, et al. Adjuvant platinum-based chemotherapy for borderline serous ovarian tumors with invasive implants. Gynecol Oncol. 2014;132:23–27. doi: 10.1016/j.ygyno.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Sneige N, Thomison JB, Malpica A, et al. Peritoneal washing cytologic analysis of ovarian serous tumors of low malignant potential to detect peritoneal implants and predict clinical outcome. Cancer Cytopathol. 2012;120:238–244. doi: 10.1002/cncy.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anfinan N, Sait K, Ghatage P, et al. Ten years experience in the management of borderline ovarian tumors at Tom Baker Cancer Centre. Arch Gynecol Obstet. 2011;284:731–735. doi: 10.1007/s00404-010-1713-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein NS, Ceniza N. Ovarian micropapillary serous borderline tumors. Clinicopathologic features and outcome of seven surgically staged patients. Am J Clin Pathol. 2000;114:380–386. doi: 10.1093/ajcp/114.3.380. [DOI] [PubMed] [Google Scholar]
- 9.Lenhard MS, Mitterer S, Kümper C, et al. Long-term follow-up after ovarian borderline tumor: Relapse and survival in a large patient cohort. Eur J Obstet Gynecol Reprod Biol. 2009;145:189–194. doi: 10.1016/j.ejogrb.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Deffieux X, Morice P, Camatte S, et al. Results after laparoscopic management of serous borderline tumor of the ovary with peritoneal implants. Gynecol Oncol. 2005;97:84–89. doi: 10.1016/j.ygyno.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Kokawa K, Mikami Y, Sakata H, et al. Clinical outcome and prognostic factors in borderline tumors of the ovary. Results from 17 years’ experience in the Kinki District of Japan (1990-2006) Eur J Gynaecol Oncol. 2009;30:155–161. [PubMed] [Google Scholar]
- 12.Wong HF, Low JJ, Chua Y, et al. Ovarian tumors of borderline malignancy: A review of 247 patients from 1991 to 2004. Int J Gynecol Cancer. 2007;17:342–349. doi: 10.1111/j.1525-1438.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 13.Park JY, Kim DY, Kim JH, et al. Surgical management of borderline ovarian tumors: The role of fertility-sparing surgery. Gynecol Oncol. 2009;113:75–82. doi: 10.1016/j.ygyno.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Laurent I, Uzan C, Gouy S, et al. Results after conservative treatment of serous borderline tumors of the ovary with a micropapillary pattern. Ann Surg Oncol. 2008;15:3561–3566. doi: 10.1245/s10434-008-0159-9. [DOI] [PubMed] [Google Scholar]
- 15.Camatte S, Morice P, Pautier P, et al. Fertility results after conservative treatment of advanced stage serous borderline tumour of the ovary. BJOG. 2002;109:376–380. doi: 10.1111/j.1471-0528.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 16.Morice P, Camatte S, Rey A, et al. Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann Oncol. 2003;14:592–598. doi: 10.1093/annonc/mdg173. [DOI] [PubMed] [Google Scholar]
- 17.Zanetta G, Rota S, Chiari S, et al. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: A prospective study. J Clin Oncol. 2001;19:2658–2664. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 18.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gershenson DM, Silva EG. Serous ovarian tumors of low malignant potential with peritoneal implants. Cancer. 1990;65:578–585. doi: 10.1002/1097-0142(19900201)65:3<578::aid-cncr2820650332>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.de Nictolis M, Montironi R, Tommasoni S, et al. Serous borderline tumors of the ovary. A clinicopathologic, immunohistochemical, and quantitative study of 44 cases. Cancer. 1992;70:152–160. doi: 10.1002/1097-0142(19920701)70:1<152::aid-cncr2820700125>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Bell KA, Kurman RJ. A clinicopathologic analysis of atypical proliferative (borderline) tumors and well-differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol. 2000;24:1465–1479. doi: 10.1097/00000478-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Akeson M, Zetterqvist BM, Dahllöf K, et al. Population-based cohort follow-up study of all patients operated for borderline ovarian tumor in western Sweden during an 11-year period. Int J Gynecol Cancer. 2008;18:453–459. doi: 10.1111/j.1525-1438.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy AW, Hart WR. Ovarian papillary serous tumors of low malignant potential (serous borderline tumors). A long-term follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer. 1996;78:278–286. doi: 10.1002/(SICI)1097-0142(19960715)78:2<278::AID-CNCR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Snider DD, Stuart GC, Nation JG, et al. Evaluation of surgical staging in stage I low malignant potential ovarian tumors. Gynecol Oncol. 1991;40:129–132. doi: 10.1016/0090-8258(91)90103-c. [DOI] [PubMed] [Google Scholar]
- 25.Prat J, De Nictolis M. Serous borderline tumors of the ovary: A long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26:1111–1128. doi: 10.1097/00000478-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Longacre TA, McKenney JK, Tazelaar HD, et al. Ovarian serous tumors of low malignant potential (borderline tumors): Outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707–723. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 27.Eichhorn JH, Bell DA, Young RH, et al. Ovarian serous borderline tumors with micropapillary and cribriform patterns: A study of 40 cases and comparison with 44 cases without these patterns. Am J Surg Pathol. 1999;23:397–409. doi: 10.1097/00000478-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Fauvet R, Demblocque E, Morice P, et al. Behavior of serous borderline ovarian tumors with and without micropapillary patterns: Results of a French multicenter study. Ann Surg Oncol. 2012;19:941–947. doi: 10.1245/s10434-011-2039-y. [DOI] [PubMed] [Google Scholar]
- 29.Kane A, Uzan C, Gouy S, et al. Fertility results and outcomes after pure laparoscopic management of advanced-stage serous borderline tumors of the ovary. Fertil Steril. 2010;94:2891–2894. doi: 10.1016/j.fertnstert.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 30.De Iaco P, Ferrero A, Rosati F, et al. Behaviour of ovarian tumors of low malignant potential treated with conservative surgery. Eur J Surg Oncol. 2009;35:643–648. doi: 10.1016/j.ejso.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Bell KA, Smith Sehdev AE, Kurman RJ. Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol. 2001;25:419–432. doi: 10.1097/00000478-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Hart WR. Borderline epithelial tumors of the ovary. Mod Pathol. 2005;18(suppl 2):S33–S50. doi: 10.1038/modpathol.3800307. [DOI] [PubMed] [Google Scholar]
- 33.Chemotherapy in advanced ovarian cancer: An overview of randomised clinical trials. Advanced Ovarian Cancer Trialists Group. BMJ. 1991;303:884–893. doi: 10.1136/bmj.303.6807.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen DG, Baak J, Belpomme D, et al. Advanced epithelial ovarian cancer: 1993 consensus statements. Ann Oncol. 1993;4(suppl 4):83–88. doi: 10.1093/annonc/4.suppl_4.s83. [DOI] [PubMed] [Google Scholar]
- 35.Trimble EL. The NIH Consensus Conference on Ovarian Cancer: Screening, treatment, and follow-up. Gynecol Oncol. 1994;55:S1–S3. doi: 10.1006/gyno.1994.1332. [DOI] [PubMed] [Google Scholar]
- 36.Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol. 2000;78:269–274. doi: 10.1006/gyno.2000.5926. [DOI] [PubMed] [Google Scholar]
- 37.Winter WE, III, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 38.Fauvet R, Boccara J, Dufournet C, et al. Restaging surgery for women with borderline ovarian tumors: Results of a French multicenter study. Cancer. 2004;100:1145–1151. doi: 10.1002/cncr.20098. [DOI] [PubMed] [Google Scholar]
- 39.du Bois A, Ewald-Riegler N, de Gregorio N, et al. Borderline tumours of the ovary: A cohort study of the Arbeitsgmeinschaft Gynäkologische Onkologie (AGO) Study Group. Eur J Cancer. 2013;49:1905–1914. doi: 10.1016/j.ejca.2013.01.035. [DOI] [PubMed] [Google Scholar]