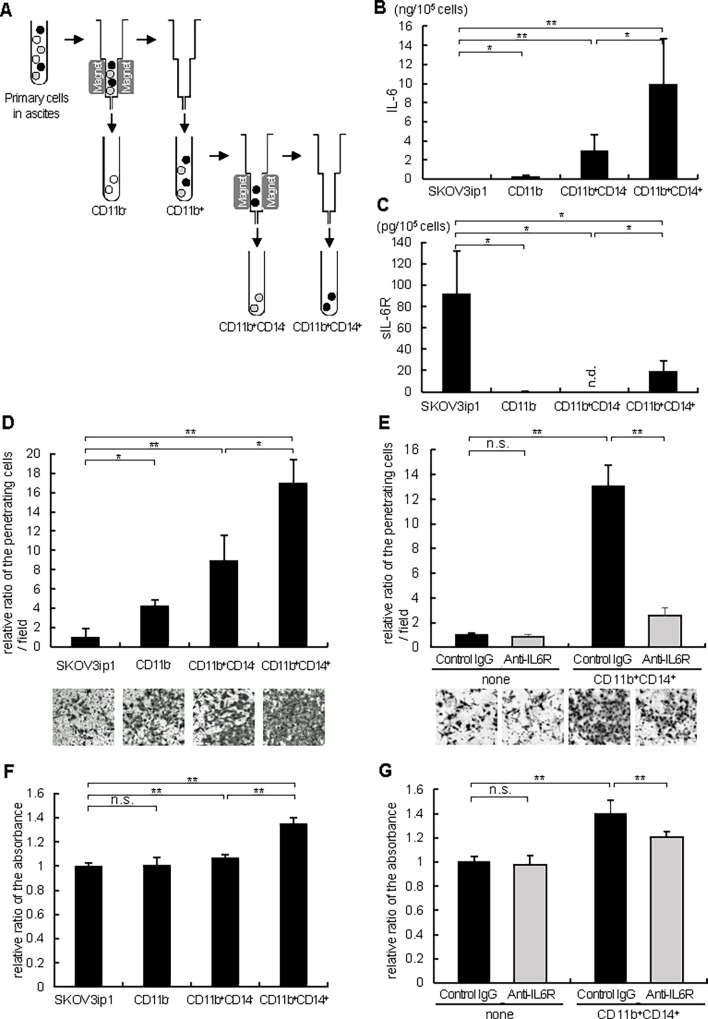
Fig 5. CD11b+CD14+ cells from ovarian cancer ascites promote ovarian cancer cell invasion and proliferation via producing IL-6.
(A) The protocol of isolation of CD11b-, CD11b+CD14- and CD11b+CD14+ cells using magnetic-activated cell sorting (MACS) technology (Miltenyi Biotech). ELISA assay of IL-6 (B) and sIL-6R (C). 1 x 105 of SKOV3ip1 cells and primary cells indicated in the figure were plated onto 6-well plates and cultured with 2 ml of serum-free medium for 72 h. Conditioned media were collected and the concentrations of human IL-6 (B) as well as sIL-6R (C) were measured by ELISA. Experiments were repeated three times and values are means (±SD) of triplicates. (D) Matrigel invasion assay. 1 x 105 SKOV3ip1 cells were placed on the upper chamber with the same number of primary cells indicated in the figure seeded on the bottom chamber as a chemoattractant, and were allowed to invade for 72 h. The relative number of invading cells when no cells were plated on the bottom chamber was set as 1.0. (E) Anti-IL-6R antibody inhibited ovarian cancer cell invasion induced by CD11b+CD14+ cells. In this experiment, the co-culture experiment in Fig. 4D was repeated with the addition of the 10 μg/ml of anti-IL-6R antibody or non-immune IgG in the bottom chamber. Representative pictures of transwells are shown in Fig. 4D and 4E (bottom). (F) In vitro cell proliferation assay. 1 x 104 SKOV3ip1 cells were plated in 24-well plates. Thereafter, polycarbonate filters with 1-μm pores were placed onto 24-well plates and the same number of primary cells indicated in the figure were seeded as a stimulant and cells were cultured for 72 h. Cell proliferation was expressed as the ratio of the number of viable cells. (G) Anti-IL-6R antibody inhibited ovarian cancer cell proliferation induced by CD11b+CD14+ cells. In this experiment, the co-culture experiment in Fig. 4F was repeated with the addition of the 10 μg/μl of anti-IL-6R antibody or non-immune IgG in the upper chamber. Experiments were repeated three times and values are means ± SD of triplicates. n.s.; not significant, n.d.; not detected, *; P < 0.05, **; P < 0.01.