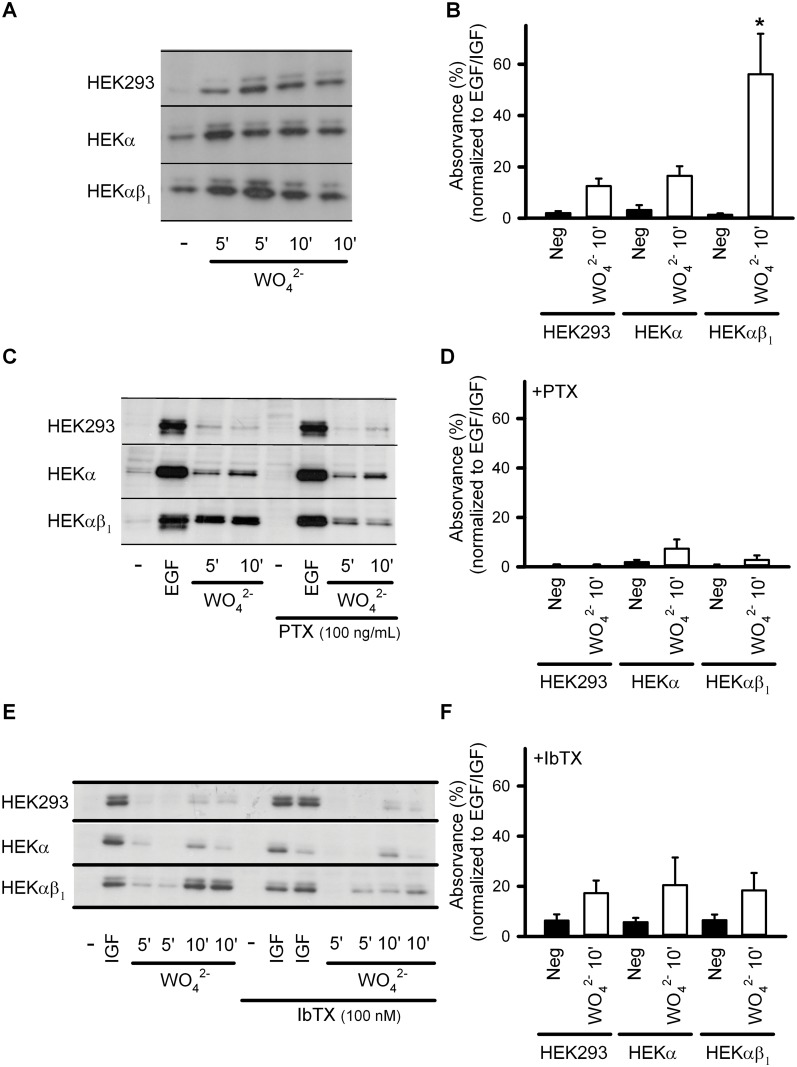
Fig 1. BKαβ1 channels potentiate tungstate-induced ERK1/2 phosphorylation in a Gi/o protein-dependent manner.
Phosphorylation of ERK1/2 was analyzed by Western blot using phospho-ERK-specific antibodies. Total ERK was used as loading control (data not shown). Protein expression and phosphorylation were quantified by densitometry of the corresponding Western blot signal. Relative density (phosphorylated versus total ERK) was normalized to the inner control (EGF/IGF for each condition, which was considered as 100%). (A) Representative Western blots obtained from HEK293, HEKα and HEKαβ1 cells for ERK1/2 phosphorylation levels, without treatment (-) or after treatment with 1 mM tungstate (WO4 2-) (during 5 and 10 minutes, as indicated) in the absence of toxins. (B) Average normalized relative density (phosphorylated versus total ERK) in the absence of toxins. (C) Representative Western blots obtained from HEK293, HEKα and HEKαβ1 cells for ERK1/2 phosphorylation levels, without treatment (-), after treatment with 100 ng/ml EGF (during 10 minutes) (EGF) or after treatment with 1 mM tungstate (WO4 2-) (during 5 and 10 minutes, as indicated) in the absence (left) or presence of PTX (right). (D) Average normalized relative density (phosphorylated versus total ERK) in the presence of PTX. (E) Representative Western blots obtained from HEK293, HEKα and HEKαβ1 cells for ERK1/2 phosphorylation levels, without treatment (-), after treatment with 100 ng/ml IGF (during 10 minutes) (IGF) or after treatment with 1 mM tungstate (WO4 2-) (during 5 and 10 minutes, as indicated) in the absence (left) or presence of IbTX (right). (F) Average normalized relative density (phosphorylated versus total ERK) in the presence of IbTX. n = 4–12 in each experimental group. *P < 0.05 when compared to the other HEK cell lines (Kruskal-Wallis test followed by Dunn post hoc test). See Methods for further details.