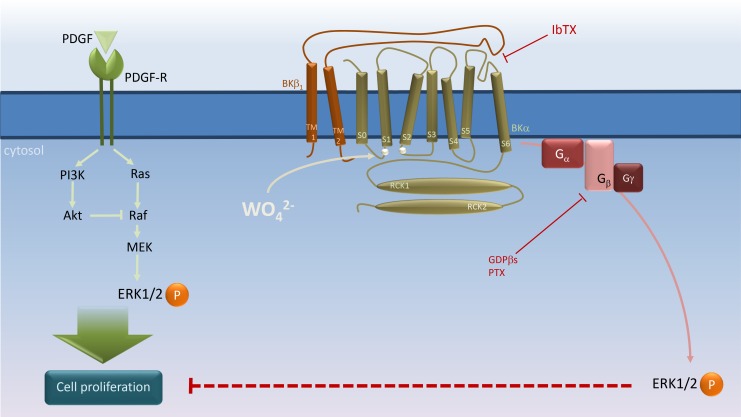
Fig 6. Schematic representation of BKαβ1 channel’s role in tungstate-induced, Gi protein-mediated ERK phosphorylation and its interaction with PDGF-stimulated cell proliferation in human vascular smooth muscle.
PDGF stimulates both the Ras/Raf/MEK/ERK and the phosphatidylinositol 3-kinase (PI3K)/Akt mitogenic signaling pathways to induce vascular smooth muscle cell (VSMC) proliferation [35]. Tungstate (WO4 2-) binding to the BK channel α subunit at a site involving residues of the Voltage Sensor Domain (silver circles in the S0–S1 and S2–S3 linkers) [14, 26] promotes activation of the voltage sensor and channel gating in a β1 subunit-dependent manner, and subsequently, activation of PTX-sensitive G proteins to induce ERK phosphorylation and inhibition of PDGF-stimulated VSMC proliferation. Binding of iberiotoxin (IbTX) to the external mouth of the channel in close proximity to the β1 extracellular loop, which plays an important role in the modulation of voltage sensor activation and gating of the BK channel [21, 46–53], may impair the conformational changes in the BKαβ1 channel produced by tungstate to prevent the coupling of channel gating to G protein activation without the need of K+ conduction. For further details, please see Discussion.