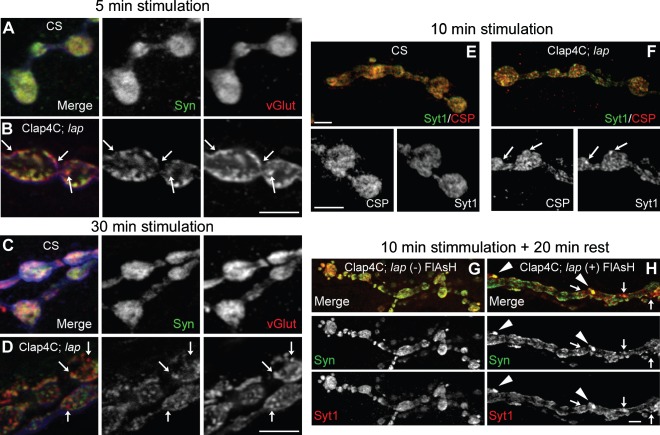
Figure 6. Independent diffusion of SV proteins following nerve stimulation is sustained for long periods following LAP inactivation.
Third instar larval NMJ preparations from the indicated genotypes were dissected, incubated with FlAsH-EDT2 and muscle 4 from segment A2 or A3 was exposed to 488-nm-filtered fluorescent light for 5 min. Clap4C; lap = Clap4C; lap1/lapsd3. Following illumination, the larval NMJs were stimulated continuously with high K+ (90 mm) Jan's solution for 5 min (A and B) or 30 min (C and D) or 10 min (E and F). Following nerve stimulation, the larvae were washed rapidly with Ca2+-free HL-3 saline and fixed. The NMJs were then stained for a pair of SV proteins Syn and vGlut or Syt1 and CSP. Panels A–D also show HRP-stained NMJs (blue) to mark the neuronal membrane. The stimulation paradigm does not affect SV protein colocalization in wild-type control larval NMJs in which most SV proteins are colocalized although we note that not all SV proteins are fully colocalized even in the wild-type larval NMJs. In contrast, there are more punctate in LAP-inactivated synaptic boutons and SV proteins are often not colocalized in these punctate (arrows). G and H) Following 10 min of stimulation with high K+, nerve terminals were allowed to rest in Ca2+-free HL-3 saline for an additional 20 min prior to fixation. Following the resting period, dramatically large punctate can be observed positive for both Syt1 and Syn (arrowheads) in larval NMJs whose LAP is inactivated, while some portions of the punctate within the bouton are positive for only one of the two SV proteins (arrows). The protocol for both control and experimental samples was identical. Scale bars = 10 µm.