Scheme 2.
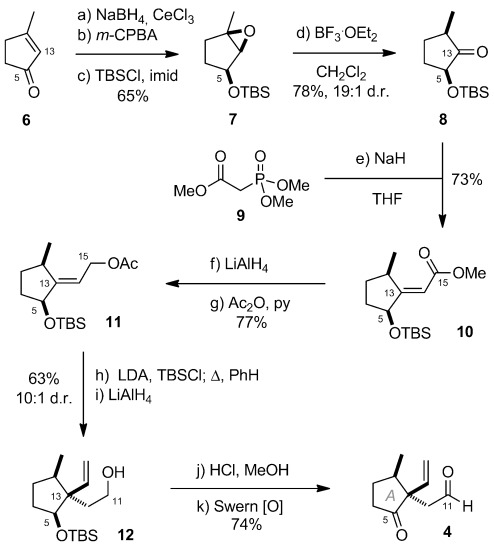
Preparation of A-ring keto-aldehyde 4. Reagents and conditions: a) NaBH4, CeCl3, MeOH, −78 °C to 0 °C; b) m-CPBA, CH2Cl2, 0 °C; c) TBSCl, imid, CH2Cl2, 0 °C to RT, 65 % over 3 steps; d) BF3⋅OEt2, CH2Cl2, −20 °C, 78 %, 19:1 d.r.; e) NaH, RT, 72 h, 73 %, >19:1 (E):(Z); f) LiAlH4, Et2O, 0 °C; g) Ac2O, py, DMAP, CH2Cl2, RT, 77 % over 2 steps; h) LDA, TBSCl, THF, −78 °C to RT; PhH, reflux, 16 h, 10:1 d.r.; i) LiAlH4, Et2O, 0 °C, 63 % over 2 steps; j) 3 n HCl, MeOH, (1:3), RT; k) DMSO, (COCl)2, Et3N, CH2Cl2, −78 °C to RT, 74 % over 2 steps. m-CPBA=3-chloroperoxybenzoic acid, DMAP=N,N-dimethyl-4-aminopyridine, DMSO=dimethylsulfoxide, imid=imidazole, LDA=lithium diisopropylamide, py=pyridine, TBS=tert-butyldimethylsilyl, THF= tetrahydrofuran.
