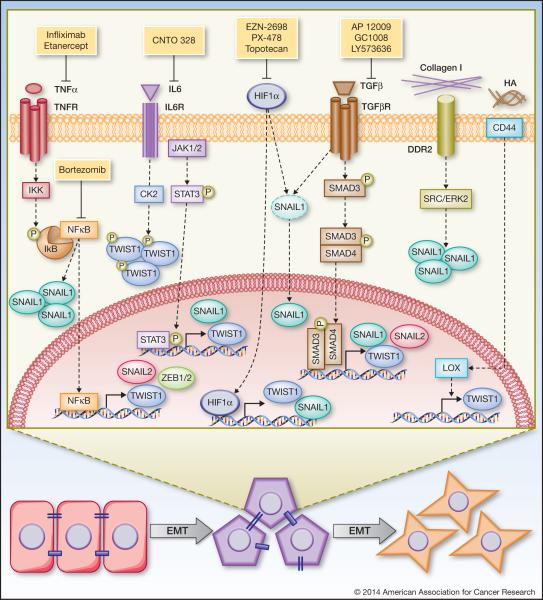
Figure 1. Regulation of EMT transcription factors by tumor microenvironmental signals.
Transforming growth factor-β (TGFβ) regulates up-regulation of TWIST1, SNAIL1, and SNAIL2 via the SMAD signaling pathway. Drugs that inhibit TGFβ are AP 12009, GC1008, LY573636, which are in clinical trial for advanced solid tumors. Tumor necrosis factors-α (TNFα) activates NFκB to induce TWIST1, SNAIL2, and ZEB1/2 expression and TNFα/NFκB activation also increases SNAIL1 protein stability. Therapeutic approaches to inhibit TGFβ signaling include TNFα antagonist (infliximab and etanercept) and NFkB inhibitor (bortezomib), all of which have been assessed in phase II clinical trial for several cancer types. Interleukin-6 (IL6) induces TWIST1 and SNAIL1 expression via JAK/STAT3 signaling and increases TWIST1 stability through CK2-dependent phosphorylation. An IL6 ligand-blocking antibody, CNTO 328, has been tested in phase I/II clinical trials with metastatic renal cell carcinoma. Hypoxia inducible factor-1α (HIF1α) induces TWIST1 and SNAIL1 expression and HIF1α either alone or in cooperation with TGFβ promotes SNAIL1 nuclear localization to stabilize SNAIL. Agents to inhibit HIF1α include EZN-2698, PX-478, and topotecan. Topotecan has been tested in phase I/II clinical trials in combination with conventional chemotherapy and EZN-2698 and PX-478 are currently being tested in phase I clinical trial. Collagen I can promote SNAIL1 stability through binding to its receptor DDR2 and activating SRC/ERK2 pathway. Hyaluronan (HA) binding to CD44 induces nuclear translocation of CD44 to directly induce Lysyl-Oxidase (LOX) expression, which in turn increases TWIST1 expression.