Abstract
Objective: Determine the percentage of subjects taking antipsychotics who meet criteria for metabolic syndrome based on point-of-care testing analyses. Evaluate pharmacist comprehensive medication management services using point-of-care tests to reduce the mean difference in number of metabolic syndrome risk parameters at 6 and 12 months.
Method: This 12-month, prospective, multisite, randomized, controlled study included 120 subjects taking antipsychotics (mean [SD] age of 42.9 [11.3] years) recruited from 3 community mental health clinics in Minnesota. Subjects consented to receive either pharmacist (PCS; n = 60) or no pharmacist (NCS; n = 60) comprehensive medication management services. Data were collected from February 2010 to January 2012.
Results: No statistical differences in metabolic syndrome based on point-of-care tests were observed between the 2 groups at baseline (PCS: 85.2%, n = 46 versus NCS: 71.2%, n = 42, P = .073) or at 12 months (PCS: 84.4%, n = 38 versus NCS: 70.2%, n = 33, P = .104). Subjects, overall, screened positive at baseline for dyslipidemia (85.8%, n = 106), hypertension (52.5%, n = 63), and diabetes (22.5%, n = 27) based on point-of-care testing for metabolic risk criteria. After 12 months, a nonsignificant (P = .099) higher adjusted mean number of metabolic syndrome parameters in PCS subjects compared to NCS subjects (mean difference [95% CI] = 0.41 [−0.08 to 0.90]) were found.
Conclusions: A relatively high proportion of subjects met criteria for metabolic syndrome, although no significant improvement was observed between the groups after 12 months. Point-of-care test analyses identified a high proportion of subjects meeting criteria for dyslipidemia, hypertension, and diabetes. Utilizing point-of-care tests in mental health settings and fostering interprofessional partnerships with comprehensive medication management pharmacists may improve identification and long-term management of metabolic risks among patients prescribed antipsychotics.
Trial Registration: ClinicalTrials.gov identifier: NCT02029989
Clinical Points
■ Current evidence demonstrates that a high proportion of patients with severe persistent mental illness who are taking antipsychotics have metabolic syndrome and are at risk for developing dyslipidemia, hypertension, diabetes, and coronary artery disease.
■ Medications classified as antipsychotics and severe persistent mental illness should be included as secondary causes of metabolic syndrome and related risks in the primary care provider’s differential diagnosis.
■ Current evidence best supports an interprofessional approach, including pharmacists, for routine metabolic screening, monitoring, and comprehensive medication management for all patients with severe persistent mental illness who are prescribed antipsychotics.
It is well-recognized in psychiatry that patients with mental illness who take antipsychotic agents continue to be affected by a severe health disparity due to lack of adequate metabolic monitoring established by consensus recommendations in 2004, which include body weight and body mass index (BMI) (baseline, monthly for 3 months, then quarterly), waist circumference (baseline and annually), blood pressure (baseline, 12 weeks, and annually), and fasting glucose and fasting lipid profile (baseline, 12 weeks, and every 5 years or annually if warranted).1–7 The medical community at-large has not been able to adequately screen and monitor these patients; even the 2013 American College of Cardiology/American Heart Association cholesterol treatment guidelines do not indicate antipsychotics or severe persistent mental illness as secondary causes of hyperlipidemia encountered in clinical practice.8 A major health care concern is the life-expectancy decrease of ∼ 25 years for patients with severe persistent mental illness, such as schizophrenia, compared with the general population.9 Equally concerning is that patients with severe persistent mental illness continue to have inadequate integration of care between psychiatry and medicine.10
Antipsychotic agents, which represent the primary treatment for many people with severe persistent mental illness, increase the risk of metabolic syndrome by about 2-fold in males and 3-fold in females compared with those without severe persistent mental illness.11–15 Suicide, medication nonadherence, metabolic syndrome due to antipsychotic side effects, and associated chronic medical illnesses contributing to sudden death are thought to be factors for the decreasing life expectancy in this population.16–19 Nevertheless, psychiatry and medical providers continue to have difficulty addressing the medication-related and medical issues that affect their patients.3,6,7,20–26 In an attempt to address these issues, the National Committee for Quality Assurance has developed Healthcare Effectiveness Data and Information Set (HEDIS) measures to analyze the performance of health care systems with regard to diabetes screening and diabetes/cardiovascular monitoring rates of patients taking antipsychotic agents.27
There has been much emphasis on interventions to address weight gain associated with antipsychotics; however, much less evidence is available with regard to consistently screening for and managing diabetes, dyslipidemia, and hypertension in people with serious mental illness.28 There are best-practice examples in the Veterans Administration and in some states, such as Minnesota, regarding the utilization of psychiatrists, case management, and psychiatric nurse practitioners to increase the patient’s access to primary care provider services.29,30 Although these approaches improve metabolic monitoring, they are limited in scope. Utilization of other health care providers such as pharmacists might address the critical shortage of mental health providers and provide the necessary link to primary care services.31
Because of the difficulty involved in getting patients with mental illness to primary care clinics or the phlebotomy laboratory, the addition of capillary blood, point-of-care tests to monitor glucose and lipid levels in community mental health centers may prove beneficial. It is highly likely that additional metabolic screenings will lead to earlier identification of new metabolic abnormalities and improved treatment for metabolic syndrome, diabetes, and/or hypertension.32,33 Also, providing comprehensive medication management would ensure that prescribed medications are effective for identified medical or psychiatric conditions, safe (based on patient physiology, comorbidities, and drug interactions), and correctly self-administered as intended (medication adherence).34 It is hypothesized that if metabolic abnormalities are identified, then providing pharmacist comprehensive medication management consultative services would reduce medication-related problems by improving medication adherence, coordination of care between psychiatry and primary care provider services, and outcomes in metabolic indices.34–40
The primary purpose of this study was to determine the percentage of subjects taking antipsychotic agents who meet criteria for metabolic syndrome at baseline using point-of-care test results. Secondary objectives included the following: (1) evaluate the effectiveness of the provision by pharmacist comprehensive medication management services regarding their ability to reduce the mean difference in number of metabolic syndrome risk parameters based on point-of-care test results at 6 and 12 months41–44 and (2) evaluate the overall impact of psychiatric medication therapy on metabolic risk.
METHOD
Design Overview
The study was a 12-month, prospective, multisite, randomized, controlled research design that comprised subjects recruited from 3 community mental health clinic settings in Minnesota (ClinicalTrials.gov identifier: NCT02029989). Data were collected from February 2010 to January 2012. A block randomization schedule was used to ensure balanced treatment assignments of subjects recruited at each site (Human Development Center, Duluth [www.humandevelopmentcenter.org]; Range Mental Health Center, Hibbing [http://www.rangementalhealth.org]; and Family Life Mental Health Center, Coon Rapids [http://www.flmhc.org]), with subjects assigned to receive either pharmacist (PCS) or no pharmacist (NCS) comprehensive medication management services. A centralized call-in system was used to inform the investigators of the subject’s random group assignment. The inclusion criteria consisted of (1) current antipsychotic therapy, (2) English speaking, (3) at least 18 years of age, (4) competent to understand and make medical choices independently, and (5) not currently or previously seen by a comprehensive medication management pharmacist. The study methods and informed consent procedures were approved by the University of Minnesota, Human Research Protection Program, Institutional Review Board (IRB) and Expedited Review Committee (IRB code 090M72212). Researchers used a proprietary software company (Medication Management Systems, Inc45) electronic medical record platform for data collection on all subjects.
Measures
The pharmacist researchers were certified Minnesota Medication Therapy Management Services providers who were trained to administer point-of-care tests and provide comprehensive medication management services.46 Each research site was equipped with point-of-care testing equipment including Omron electronic blood pressure monitors (HEM-790IT),47 Health o meter body weight scales (500KL),48 and waist circumference measuring tapes. Clinical Laboratory Improvement Amendments (CLIA) waivers were obtained to perform capillary blood sampling. The validated instruments used included the Cholestech LDX glucose/lipid and glycosylated hemoglobin A1c Now devices.49–51 The number of study visits for the subjects receiving PCS varied between 5 and 7 follow-up visits during the year of the study depending on the pharmacist’s judgment. In contrast, NCS subjects were assessed only at baseline and 6-month and 12-month study visits to review medications and diagnoses and obtain point-of-care test results. Interpretation of point-of-care test results, care plans, or recommendations were not provided to NCS subjects unless patient safety issues were identified.
For point-of-care test analyses, diagnostic criteria and goal values for metabolic syndrome and other metabolic risk parameters were established by the American Diabetes Association 2013, National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III), American Heart Association, National Heart Lung and Blood Institute, American Association of Clinical Endocrinologists, and Joint National Committee 7.42–44,52 Metabolic syndrome was defined as meeting any 3 of the following criteria: fasting glucose > 100 mg/dL or diagnosed with diabetes mellitus; blood pressure: systolic/diastolic ≥ 130/≥ 85 mm Hg or antihypertensive treatment; high-density lipoprotein (HDL): men < 40 mg/dL, women < 50 mg/dL; triglycerides > 150 mg/dL; and central obesity: men > 40 inches, women > 35 inches. Other point-of-care tests for metabolic risk criteria included BMI > 26 calculated as kg/m2; total cholesterol (fasting): 200–239 mg/dL (borderline high); low-density lipoprotein (LDL) (fasting): 130–159 mg/dL (borderline high); hip/waist ratios: men > 0.90, women > 0.85; glycosylated hemoglobin (A1c) for diabetes ≥ 6.5% and prediabetes 5.7%–6.4%; and Framingham risk scores (10-year risk percentage for a coronary heart disease event for men and women). A correction factor (−3.7%/nonfasting hours) for nonfasting triglycerides (ie, capillary samples obtained ≥ 2 hours postprandial) was used in the calculation of corrected triglyceride levels, corrected total cholesterol levels, and corrected Framingham risk scores.53
Statistical Analysis
The Biostatistical Design and Analysis Center in the Clinical and Translational Science Institute at the University of Minnesota, Minneapolis (www.ctsi.umn.edu), provided the main statistical consultation support for the primary and secondary objectives. The study sample size enrollment goal per group was determined a priori to be 105 subjects (total N = 210) and was based on a power analysis to achieve greater than 80% power to detect, at α = .05, a 15%–20% absolute percent difference between the PCS and NCS groups in the proportion of subjects with increased risk of metabolic syndrome at 6 and 12 months.
Descriptive statistics used to summarize baseline and 12-month results included number (percent) for categorical variables and mean (SD) or median (minimum, maximum) for continuous variables. Baseline and 12-month between-group comparisons were done using χ2 tests for categorical variables and 2-sample t tests for continuous variables. Between-group comparisons of mean difference in the number of metabolic syndrome risk parameters at 6 and 12 months adjusted for site and baseline measures were done using multiple linear regression models, with results reported as mean differences (95% confidence intervals). The metabolic risks associated with the psychiatric medications were determined by taking the sum of the psychiatric medication metabolic risk scores partially derived from the antipsychotic risk severities described in the 2004 consensus development conference and clinical practice.12 The mean of the summative scores was calculated and compared across the PCS and NCS groups (eg, a subject taking olanzapine [3 = high risk] and aripiprazole [1 = low metabolic risk] has a risk score total = 4).
RESULTS
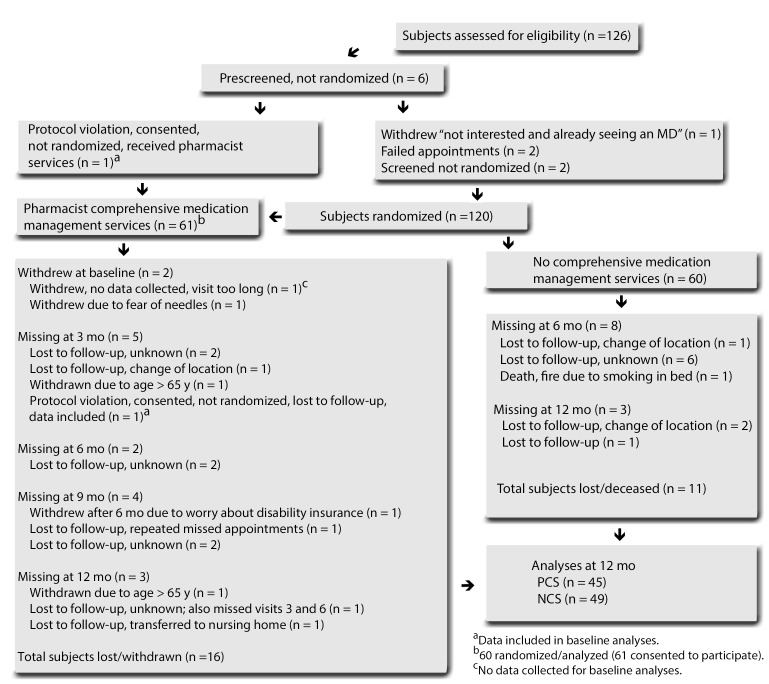
A total of 120 subjects (121 subjects consented to participate) were randomized to either PCS or NCS, 60 in each group. All subjects (N = 120) were analyzed throughout the study with point-of-care tests regardless of previous diagnostic conditions (ie, dyslipidemia, hypertension, and diabetes) for the purpose of determining which subjects were at their goal in the management of their condition as well as monitoring subjects without previous conditions. Sixty PCS subjects received baseline metabolic screening, but only 58 of the PCS randomized subjects received pharmacist comprehensive medication management services (Figure 1). Only 94 subjects completed the final 12-month visit, 45 in the PCS group and 49 in the NCS group. The flow diagram (Figure 1) summarizes the number of subjects who dropped out prior to the final 12-month study visit as well as reasons for drop-out.
Figure 1.
Flow Diagram of Study Subjects
During the study, 1 NCS subject who received comprehensive medication management services (due to excessively high triglyceride levels) was reported as a protocol violation to the IRB. Other protocol violations included in the data analyses involved almost half (n = 28) of NCS subjects receiving some degree of comprehensive medication management services (ie, identified drug therapy problems) determined from separate reports provided by Medication Management Systems, Inc.
The overall baseline characteristics of the study population indicate a majority were white (86.7%, n = 104), female (59.2%, n = 71), unemployed (79.2%, n = 95), and unmarried (82.5%, n = 99), with an overall mean (SD) age of 42.9 (11.3) years. Overall baseline rates for DSM-IV-TR psychiatric diagnoses included anxiety disorders (76.7%, n = 89) (including posttraumatic stress disorder [n = 12] and obsessive-compulsive disorders [n = 3]), depressive disorders (65.8%, n = 79), bipolar disorders (47.5%, n = 57), schizophrenia (30.8%, n = 37), and schizoaffective disorder (22.5%, n = 27). Overall baseline rates for dyslipidemia, hypertension, and diabetes were 57.5% (n = 69), 37.5% (n = 45), and 20.8% (n = 25), respectively. Most (79%, n = 94) of the subjects were sedentary or engaged in only light physical activities. Other baseline results include primary care visit within 12 months prior to baseline: 68.3% (n = 82); self/family report history of dyslipidemia: 47.5% (n = 57)/56.7% (n = 68); self/family report history of hypertension: 41.7% (n = 50)/65% (n = 78); self/family report history of diabetes: 32.5% (n = 39)/67.5% (n = 81); tobacco use: 52.9% (n = 63), with smokers precontemplative for smoking cessation: 38.1% (n = 24); alcohol use (> 2 drinks/wk): 9.2% (n = 11), history of alcohol use disorder: 0.8% (n = 1), and other substance use disorders: 2.5% (n = 3).
The percentage (number) of subjects with metabolic conditions at baseline included 58.3% (n = 70) with dyslipidemia, 44.2% (n = 53) with hypertension, and 22.5% (n = 27) with diabetes. The overall mean (SD) baseline results from the point-of-care tests included systolic/diastolic blood pressure: 118 (18.9)/81.9 (12.7) mm Hg; BMI: 34.3 (8.74); combined male and female waist/hip measurements: 44.5 (7.29)/46.2 (6.97) inches; glucose: 124 (46.2) mg/dL; glycosylated hemoglobin A1c: 5.7% (1.08%); corrected total cholesterol: 192 (42.5) mg/dL; LDL: 106 (35.3) mg/dL; corrected triglycerides: 229 (149.5) mg/dL; and HDL: 40.8 (13.0) mg/dL. A majority of subjects (73.3%, n = 88) were identified with metabolic syndrome at baseline.
Primary Objective Results
Based on point-of-care test results, there was no difference in metabolic syndrome, abdominal obesity, dyslipidemia, hypertension, and diabetes between the 2 groups at baseline and at 12 months (Table 1). Likewise, most PCS and NCS subject group characteristics were not significantly different at baseline including metabolic syndrome indices (ie, blood pressure, BMI, waist/hip circumference, cholesterol, and diabetes). In contrast, the proportion of subjects identified with dyslipidemia was PCS: 76.7% (n = 46) versus NCS: 38.3% (n = 23), P < .001 at baseline and PCS: 84.4% (n = 38) versus NCS: 36.7% (n = 18), P < .001 at 12 months. The proportion of subjects identified with hypertension was PCS: 46.7% (n = 28) versus NCS: 28.3% (n = 17), P = .038 at baseline.
Table 1.
Baseline and 12-Month Point-of-Care Test Results of Subjects Meeting Metabolic Risk Criteria
| NCS |
PCS |
|||||
| Metabolic Risk Criteria | n | % | n | % | χ test P Value | Data NA |
| Baselinea | ||||||
| Metabolic syndrome | 42 | 71.2 | 46 | 85.2 | .073 | 7 |
| Waist circumference risk (men: > 40, women: > 35) | 51 | 86.4 | 50 | 87.7 | .837 | 4 |
| Hip/waist ratio risk (men: > 0 .90, women: > 0.85) | 58 | 98.3 | 57 | 100 | .324 | 4 |
| Cholesterol risk (> 200 mg/dL) | 25 | 41.7 | 22 | 38.6 | .735 | 3 |
| Corrected cholesterol risk (> 200 mg/dL) | 23 | 38.3 | 20 | 35.1 | .716 | 3 |
| LDL risk (> 130 mg/dL) | 12 | 24.5 | 13 | 25.5 | .908 | 20 |
| Triglyceride risk (> 150 mg/dL) | 32 | 54.2 | 24 | 42.1 | .191 | 4 |
| Corrected triglyceride risk (> 150 mg/dL) | 31 | 52.5 | 23 | 40.4 | .188 | 4 |
| HDL risk (men: < 40, women: < 50) | 45 | 75 | 44 | 73.3 | .835 | 7 |
| Hypertension risk (> 130/85 mm Hg) | 25 | 48.1 | 27 | 51.9 | .711 | 2 |
| Diabetes by HbA1c criteria (HbA1c ≥ 6.5) | 6 | 10.3 | 7 | 12.5 | .717 | 6 |
| Diabetes risk (HbA1c: 5.7%–6.4%) | 11 | 19.0 | 18 | 32.1 | .106 | 6 |
| 12 Monthsb | ||||||
| Metabolic syndrome | 33 | 70.2 | 38 | 84.4 | .104 | 2 |
| Waist circumference risk (men: > 40,women: > 35) | 36 | 76.6 | 38 | 84.4 | .3428 | 2 |
| Hip/waist ratio risk (men: > 0 .90, women: > 0.85) | 46 | 97.9 | 44 | 97.8 | .9752 | 2 |
| Cholesterol risk (> 200 mg/dL) | 13 | 26.5 | 13 | 28.9 | .7985 | 0 |
| Corrected cholesterol risk (> 200 mg/dL) | 13 | 26.5 | 11 | 24.4 | .8168 | 0 |
| LDL risk (> 130 mg/dL) | 5 | 11.6 | 6 | 14.6 | .6831 | 10 |
| Triglyceride risk (> 150 mg/dL) | 25 | 51.0 | 22 | 48.9 | .8364 | 0 |
| Corrected triglyceride risk (> 150 mg/dL) | 20 | 40.8 | 20 | 44.4 | .7223 | 0 |
| HDL risk (men: < 40, women: < 50) | 32 | 65.3 | 30 | 66.7 | .8894 | 0 |
| Hypertension risk (> 130/85 mm Hg) | 21 | 46.7 | 24 | 53.3 | .355 | 1 |
| Diabetes by HbA1c criteria (HbA1c ≥ 6.5) | 5 | 11.1 | 7 | 15.9 | .508 | 5 |
| Diabetes risk (HbA1c: 5.7%–6.4%) | 8 | 17.8 | 10 | 22.7 | .561 | 5 |
N = 120 (PCS: n = 60, NCS: n = 60).
n = 94 (PCS: n = 45, NCS: n = 49).
Abbreviations: Data NA = data were not available, HBA1c = glycosylated hemoglobin A1c, HDL = high-density lipoprotein, LDL = low-density lipoprotein, NCS = no pharmacist comprehensive medication management services control subject group, PCS = pharmacist comprehensive medication management services subject group.
In an effort to understand the reason for the baseline group differences in dyslipidemia and hypertension, ad hoc descriptive analyses were performed comparing point-of-care test results and likelihood of researchers identifying subjects with dyslipidemia and hypertension within both PCS and NCS groups (Table 2). The analyses revealed and it was verified that most researchers did not utilize point-of-care test results to identify dyslipidemia, hypertension, and diabetes in the NCS group. A similar proportion of subjects in each group screened positive in point-of-care testing for metabolic risk criteria for dyslipidemia (ie, PCS: 93%, n = 56 versus NCS: 83.3%, n = 50), hypertension, and diabetes at baseline (Table 2). However, NCS subjects who met point-of-care testing for metabolic risk criteria at baseline were less likely to be identified with dyslipidemia (ie, NCS: 48%, n/n = 20/50 versus PCS: 82.1%, n/n = 43/56), hypertension, and diabetes compared to the PCS subjects.
Table 2.
Baseline Comparison of Subjects Who Screened Positive With Point-of-Care Testing for Metabolic Risk Criteria and Were Identified as Having Dyslipidemia, Hypertension, and Diabetesa,b
| Baseline | Subjects With Identified Conditions or Related Medications | Subjects Who Screened Positive With Point-of-Care Testing for Metabolic Risk Criteriac | Subjects Who Screened Positive and With Identified Conditions or Related Medications |
| Dyslipidemia | |||
| Total | 58.3 (70/120) | 85.8 (106/120) | 59.4 (63/106) |
| PCS | 76.7 (46/60)* | 93.3 (56/60) | 76.8 (43/56) |
| NCS | 40.0 (24/60) | 83.3 (50/60) | 40.0 (20/50) |
| Hypertension | |||
| Total | 44.2 (53/120) | 52.5 (63/120) | 84.1 (33/63) |
| PCS | 53.3 (32/60)** | 53.3 (32/60) | 62.5 (20/32) |
| NCS | 35.0 (21/60) | 51.7 (31/60) | 41.9 (13/31) |
| Diabetes | |||
| Total | 22.5 (27/120) | 22.5 (27/120) | 55.6 (15/27) |
| PCS | 26.7 (16/60) | 25.0 (15/60) | 60.0 (9/15) |
| NCS | 18.3 (11/60) | 20.0 (12/60) | 50.0 (6/12) |
Data are presented as n (%).
Total N = 120; PCS: n = 60; NCS: n = 60.
Point-of-care testing for metabolic risk criteria not at goal with identified conditions: dyslipidemia risk (total cholesterol > 200 mg/dL or LDL > 130 mg/dL or triglycerides > 150 mg/dL or men: HDL < 40 mg/dL or women: HDL < 50 mg/dL), hypertension risk (no diabetes: > 130/80 mm Hg, diabetes: > 140/85 mm Hg), and diabetes risk: (fasting = 8 h) postprandial glucose ≥ 100 mg/dL or random (1 h, 2 h, 4 h postprandial) glucose ≥ 200 mg/dL or A1c ≥ 6.5%).
P < .001.
P = .038.
Abbreviations: HDL = high-density lipoprotein, LDL = low-density lipoprotein, NCS = no pharmacist comprehensive medication management services control subject group (no point-of-care testing data were utilized to identify conditions), PCS = pharmacist comprehensive medication management services subject group (point-of-care testing data were utilized to identify conditions).
Secondary Objectives Results
Analyses of pharmacists providing comprehensive medication management services for metabolic syndrome include results from multiple linear regression models and showed between-group differences in adjusted mean number of metabolic syndrome parameters at 6 months, which were not significant, and a nonsignificant (P = .099) higher adjusted mean number of metabolic syndrome parameters in PCS subjects compared to NCS subjects (mean difference [95% CI] = 0.41 [−0.08 to 0.90]) at 12 months (Table 3). The multiple linear regression models were adjusted for site, number of baseline identified conditions (dyslipidemia, hypertension, and diabetes), number of medications at baseline, and baseline number of metabolic syndrome parameters.
Table 3.
Adjusted Models Comparing PCS and NCS Subjects for Mean Difference in Number of Metabolic Syndrome Risk Parameters at 6 and 12 Months
| 6 Months |
12 Months |
|||
| Multiple Linear Regression | PCS Minus NCS Mean Difference (95% CI) | P Value | PCS Minus NCS Mean Difference (95% CI) | P Value |
| No. of metabolic syndrome risk parametersa | 0.24 (−0.27 to 0.75) | .364 | 0.41 (−0.08 to 0.90) | .099 |
| Adjusted for | ||||
| Research sitesb | .004 | NS | ||
| Total number of metabolic conditionsc | .016 | NS | ||
| Baseline medications | NS | NS | ||
| Baseline no. of metabolic syndrome risk parametersd | < .001 | < .001 | ||
Positive mean differences would suggest a higher mean number of metabolic syndrome risk factors in the PCS group.
Coon Rapids, Minnesota, research site had higher mean number of metabolic conditions compared to Duluth and Hibbing, Minnesota, research sites at 6 mo.
Subjects with higher total number of metabolic conditions at baseline had higher mean number of metabolic conditions at 6 mo.
Subjects meeting metabolic syndrome criteria at baseline had a higher mean number of metabolic syndrome parameters at 6 mo and 12 mo.
Abbreviations: NCS = no pharmacist comprehensive medication management services control subject group (no point-of-care testing data were utilized to identify conditions), NS = not significant, PCS = pharmacist comprehensive medication management services subject group (point-of-care testing data were utilized to identify conditions).
Analyses were done to determine the impact of psychiatric medication use including subjects taking multiple psychiatric medications associated with varying degrees of cardiovascular/metabolic risks (high risk versus low risk). Overall, there were no statistical between-group mean differences in psychiatric medication metabolic summative risk scores (Table 4).
Table 4.
Baseline and 12-Month Summative Metabolic Risk Scores With Psychiatric Medicationsa
| PCS Group (n = 60) |
NCS Group (n = 60) |
||||
| Summative Metabolic Risk Scores | Mean (SD) | Median (range) | Mean (SD) | Median (range) | t Test P Value |
| Baselineb | 2.53 (1.59) | 2 (1–9) | 2.63 (1.46) | 3 (1–7) | .72 |
| 12 Monthsc | 2.40 (1.56) | 2 (0–7) | 2.59 (1.41) | 3 (0–6) | .5346 |
Psychiatric medication metabolic risk scores (lowest −1, 0, 1, 2, 3 highest). Typical antipsychotics: haloperidol, fluphenazine, trifluoperazine, perphenazine, prochlorperazine, loxapine, molindone = 1; chlorpromazine, thioridazine = 2. Atypical antipsychotics: clozapine, olanzapine, quetiapine = 3; risperidone, iloperidone, paliperidone, asenapine = 2; lurasidone, ziprasidone, aripiprazole = 1. Serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors = 1, buspirone = 1; paroxetine, mirtazapine, vilazodone = 2; lithium/divalproex = 2; bupropion = 0; carbamazepine/oxcarbazapine, gabapentin, pregabalin, lamotrigine = 0; topiramate/zonisamide = −1. Stimulants = −1.
PCS group: n = 60; NCS group: n = 60.
PCS group: n = 45; NCS group: n = 49.
Abbreviations: NCS = no pharmacist comprehensive medication management services control subject group, PCS = pharmacist comprehensive medication management services subject group.
DISCUSSION
The results of this study are consistent with the literature and demonstrate a high proportion of patients taking antipsychotics who met criteria for metabolic syndrome and increased risk for future cardiovascular complications. The benefits of consistent metabolic screening and monitoring of patients taking antipsychotic agents are apparent from this research. Pharmacist comprehensive medication management services combined with the utilization of point-of-care screenings significantly increased the likelihood of identifying dyslipidemia and hypertension compared to the usual care (NCS) group.
Even after a decade of evidence suggesting increased metabolic syndrome risk, patients taking antipsychotics remain “under the radar” for most primary care providers and pharmacists. Mitigating factors for this include high no-show rates for appointments, acute or unresolved psychiatric symptoms, restrictions (eg, time, bureaucracy) that impede access and communication across medical and psychiatric health systems, relatively young population (ie, under 40 years of age), and the exclusion of antipsychotics and severe persistent mental illness from published lists of secondary causes of dyslipidemia in current cholesterol guidelines, all of which contribute to the lack of consistent monitoring.8 Regardless of possible reasons, there is an urgent need for a best-practice model for health care systems to improve HEDIS scores and health outcomes in this population. Ideally, the best-practice model should include seamless communication between clinicians and a strategy that integrates electronic medical records and prescribing/dispensing software to flag recommended metabolic monitoring for patients taking antipsychotics.
The utilization of pharmacist comprehensive medication management services, point-of-care test analyses, and a metabolic monitoring strategy for antipsychotic therapy, in this research study, exemplifies a best practice for primary care and mental health providers. Pharmacists who provide comprehensive medication management services are trained to foster partnerships with patients and primary care and mental health providers. Pharmacists providing comprehensive medication management services with the support of community/institution pharmacists (ie, independent, chain, hospital) can assist the primary care providers by (1) establishing therapeutic patient relationships through direct face-to-face encounters; (2) sharing responsibility for safe and effective medication outcomes; (3) assessing the patient’s medication therapy goals; (4) identifying medication-related problems; and (5) recommending patient-specific strategies to address medication-related problems and thus reducing workload burden and improving overall patient care for primary care and mental health providers.34
In summary, this is the first prospective study including pharmacist comprehensive medication management services and utilization of point-of-care screening for metabolic syndrome, metabolic risks, or related diseases in patients prescribed antipsychotics in multiple community mental health settings. The brief duration of the study may have contributed to the negative finding that the PCS subjects had no observed reduction in the mean number of metabolic risk parameters at 12 months compared to the NCS group. There are other limitations to this study. The results of a post hoc summary report provided by MMS, Inc, revealed a potential contamination bias, wherein drug therapy problems were identified in almost half of NCS subjects. The combination effects of not utilizing point-of-care testing results to identify dyslipidemia/hypertension and the contamination bias in the NCS group may have negatively affected the ability to assess the impact of comprehensive medication management services for reducing mean number of metabolic syndrome parameters. Other limitations include (1) smaller than anticipated sample size; (2) high number of subjects lost to follow-up after 12 months; (3) lack of interprofessional collaborations with primary care providers reducing the effectiveness of comprehensive medication management service impact on medication management and overreliance on facsimiles, telephone calls, and letters as the only secure methods of communication; and (4) point-of-care test risk parameters are not reflective of the recent changes in the 2013 American College of Cardiology/American Heart Association guidelines, 2012 Canadian Cardiovascular Society guidelines, or Joint National Committee 8 hypertension guidelines.8,54,55
Unfortunately, the financial unsustainability of Cholestech LDX or A1c Now screening, due to the inability of CLIA-waived community mental health centers to submit and collect claims for laboratory tests, severely limited future applications and research of point-of-care testing in these settings. However, this study provides lessons learned in the refinement and standardization of future research of pharmacist comprehensive medication management services.56–58 Separate future follow-up reports will include detailed descriptions of the pharmacist comprehensive medication management services provided to PCS and compare the ATP-III to the ATP-IV (2013) lipid guidelines based on the point-of-care test results and risk factors.
In conclusion, utilizing point-of-care testing in mental health settings and fostering interprofessional partnerships with comprehensive medication management pharmacists may improve early identification and long-term management of metabolic risks among patients prescribed antipsychotics.
Drug names: aripiprazole (Abilify), asenapine (Saphris), bupropion (Wellbutrin, Aplenzin, and others), buspirone (BuSpar and others), carbamazepine (Carbatrol, Equetro, and others), clozapine (Clozaril, FazaClo, and others), divalproex (Depakote and others), gabapentin (Neurontin and others), haloperidol (Haldol and others), iloperidone (Fanapt), lamotrigine (Lamictal and others), lithium (Lithobid and others), loxapine (Loxitane and others), lurasidone (Latuda), mirtazapine (Remeron and others), molindone (Moban), olanzapine (Zyprexa), paliperidone (Invega), paroxetine (Paxil, Pexeva, and others), pregabalin (Lyrica), prochlorperazine (Compro and others), quetiapine (Seroquel), risperidone (Risperdal and others), topiramate (Topamax and others), vilazodone (Viibryd), ziprasidone (Geodon), zonisamide (Zonegran and others).
Potential conflicts of interest: Dr Schneiderhan has received honoraria from American Society of Health System Pharmacists. Dr Shuster and Ms Davey report no conflicts of interest related to the subject of this article.
Funding/support: Funded by grant support from Medica Foundation, Minneapolis, Minnesota, and Peters Institute of Pharmaceutical Care, College of Pharmacy, University of Minnesota, Minneapolis.
Role of the sponsor: Medica Foundation or the Peters Institute of Pharmaceutical Care had no role in the study design, protocol, data collection, analyses, interpretation of the data, or approval of this article.
Acknowledgments: Administrators: Randall Seifert, PharmD, College of Pharmacy, University of Minnesota, Duluth; Peter Miller, MD, Minnesota Department of Human Services, Duluth; and Steve Bauer, MD, Human Development Center, Duluth, Minnesota. Researchers: Daniel Tomaszewski, PharmD, and Sarah Schweiss, PharmD, College of Pharmacy, University of Minnesota, Duluth; Julie Gambaiani, PharmD, Genoa Healthcare, Minneapolis, Minnesota; and Sara Madden, PharmD, Fairview Range Medical Center, Hibbing, Minnesota. Research assistant: Nicole Birch, BS, University of Minnesota. Support staff/randomization: Anna Firoozi, College of Pharmacy, University of Minnesota, Duluth. Dr Schweiss received honoraria from Sanofi for an education program. The other acknowledged individuals have no conflicts of interest to report related to the subject of this article.
References
- 1.Jennex A, Gardner DM. Monitoring and management of metabolic risk factors in outpatients taking antipsychotic drugs: a controlled study. Can J Psychiatry. 2008;53(1):34–42. doi: 10.1177/070674370805300106. [DOI] [PubMed] [Google Scholar]
- 2.Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of nonfasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 3.van Winkel R, De Hert M, Van Eyck D, et al. Screening for diabetes and other metabolic abnormalities in patients with schizophrenia and schizoaffective disorder: evaluation of incidence and screening methods. J Clin Psychiatry. 2006;67(10):1493–1500. doi: 10.4088/jcp.v67n1002. [DOI] [PubMed] [Google Scholar]
- 4.Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51(8):492–501. doi: 10.1177/070674370605100804. [DOI] [PubMed] [Google Scholar]
- 5.Fagiolini A. Medical monitoring in patients with bipolar disorder: a review of data. J Clin Psychiatry. 2008;69(6):e16. doi: 10.4088/jcp.0608e16. [DOI] [PubMed] [Google Scholar]
- 6.Morrato EH, Newcomer JW, Allen RR, et al. Prevalence of baseline serum glucose and lipid testing in users of second-generation antipsychotic drugs: a retrospective, population-based study of Medicaid claims data. J Clin Psychiatry. 2008;69(2):316–322. doi: 10.4088/jcp.v69n0219. [DOI] [PubMed] [Google Scholar]
- 7.Morrato EH, Newcomer JW, Kamat S, et al. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037–1042. doi: 10.2337/dc08-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Morbidity and mortality in people with serious mental illness. Thirteenth in a series of technical reports, vol. 13. In: Parks J, Svendsen D, Singer P, et al., editors. Alexandria, VA: National Association of State Mental Health Program Directors Medical Directors Council; 2006. pp. 4–87. http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality and Morbidity Final Report 8.18.08.pdf. Updated October 2006. Accessed January 10, 2014. [Google Scholar]
- 10.Final report for the president’s new freedom commission on mental health. Substance abuse and mental health services administration: SAMHSA’s National Mental Health Information Center; 2003. Achieving the promise: transforming mental health care in America, vol SMA 03-3832; pp. 1–84. http://govinfo.library.unt.edu/mentalhealthcommission/reports/reports.htm. Accessed January 12, 2014. [Google Scholar]
- 11.Abidi S, Bhaskara SM. From chlorpromazine to clozapine: antipsychotic adverse effects and the clinician’s dilemma. Can J Psychiatry. 2003;48(11):749–755. doi: 10.1177/070674370304801107. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association American Psychiatric Association American Association of Clinical Endocrinologists et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 13.Ananth J, Parameswaran S, Gunatilake S. Side effects of atypical antipsychotic drugs. Curr Pharm Des. 2004;10(18):2219–2229. doi: 10.2174/1381612043384088. [DOI] [PubMed] [Google Scholar]
- 14.Barnett AH, Mackin P, Chaudhry I, et al. Minimizing metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidemia. J Psychopharmacol. 2007;21(4):357–373. doi: 10.1177/0269881107075509. [DOI] [PubMed] [Google Scholar]
- 15.Meyer JM, Davis VG, McEvoy JP, et al. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE schizophrenia trial phase 1. Schizophr Res. 2008;103(1–3):104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druss BG, Walker ER. Mental disorders and medical comorbidity. Synth Proj Res Synth Rep. 2011;(21):1–26. [PubMed] [Google Scholar]
- 17.Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129–141. doi: 10.2165/00023210-200721020-00004. [DOI] [PubMed] [Google Scholar]
- 18.McGinty EE, Blasco-Colmenares E, Zhang Y, et al. Postmyocardial infarction quality of care among disabled Medicaid beneficiaries with and without serious mental illness. Gen Hosp Psychiatry. 2012;34(5):493–499. doi: 10.1016/j.genhosppsych.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd C, Leff B, Weiss C, et al. Faces of Medicaid: Clarifying multimorbidity patterns to improve targeting and delivery of clinical services for Medicaid populations. Center for Health Care Strategies. 2010 http://www.chcs.org/resource/faces-of-medicaid-clarifying-multimorbidity-patterns-to-improve-targeting-and-delivery-of-clinical-services-for-medicaid-populations/. Updated December 2010. Accessed July 29, 2014. [Google Scholar]
- 21.Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry. 2010;67(1):17–24. doi: 10.1001/archgenpsychiatry.2009.179. [DOI] [PubMed] [Google Scholar]
- 22.Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8–13. [PubMed] [Google Scholar]
- 23.Newcomer JW, Sernyak MJ. Identifying metabolic risks with antipsychotics and monitoring and management strategies. J Clin Psychiatry. 2007;68(7):e17. doi: 10.4088/jcp.0707e17. [DOI] [PubMed] [Google Scholar]
- 24.van Winkel R, De Hert M, Van Eyck D, et al. Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder. Bipolar Disord. 2008;10(2):342–348. doi: 10.1111/j.1399-5618.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 25.Wirshing DA, Pierre JM, Erhart SM, et al. Understanding the new and evolving profile of adverse drug effects in schizophrenia. Psychiatr Clin North Am. 2003;26(1):165–190. doi: 10.1016/s0193-953x(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 26.Masand PS, Culpepper L, Henderson D, et al. Metabolic and endocrine disturbances in psychiatric disorders: a multidisciplinary approach to appropriate atypical antipsychotic utilization. CNS Spectr. 2005;10(suppl 14) l14, 1–15. [PubMed] [Google Scholar]
- 27.HEDIS 2014 Measures. National Committee for Quality Assurance (NCQA) website. http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2014/List%20of%20HEDIS%202014%20Measures.pdf. Accessed January 10, 2014.
- 28.Gierisch JM, Nieuwsma JA, Bradford DW, et al. Interventions To Improve Cardiovascular Risk Factors in People With Serious Mental Illness. Rockville, MD: US Department of Health and Human Services; 2013. [PubMed] [Google Scholar]
- 29.Trangle M, Gary M, Paul G, et al. Minnesota 10 by 10. Reducing morbidity and mortality in people with serious mental illnesses. Minn Med. 2010;93(6):38–41. [PubMed] [Google Scholar]
- 30.Maki M, Bjorklund P. Improving cardiovascular disease screening in community mental health centers. Perspect Psychiatr Care. 2013;49(3):179–186. doi: 10.1111/j.1744-6163.2012.00348.x. [DOI] [PubMed] [Google Scholar]
- 31.McKee JR, Lee KC, Cobb CD. Psychiatric pharmacist integration into the medical home. Prim Care Companion CNS Disord. 2013;15(4) doi: 10.4088/PCC.13com01517. 10.4088/PCC.13com01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8):975–987. doi: 10.1592/phco.29.8.975. [DOI] [PubMed] [Google Scholar]
- 33.Batscha C, Schneiderhan ME, Kataria Y, et al. Treatment settings and metabolic monitoring for people experiencing first-episode psychosis. J Psychosoc Nurs Ment Health Serv. 2010;48(9):44–49. doi: 10.3928/02793695-20100730-03. [DOI] [PubMed] [Google Scholar]
- 34.The patient-centered medical home: integrating comprehensive medication management to optimize patient outcomes: a resource guide. Patient-Centered Primary Care Collaborative (PCPCC) website. http://www.pcpcc.org/guide/patient-health-through-medication-management. Accessed January 12, 2014.
- 35.Medication therapy management in pharmacy practice. core elements of an MTM service model version 2.0. Updated core elements released at APhA2008 website. http://www.pharmacist.com/sites/default/files/files/core_elements_of_an_mtm_practice.pdf. Updated 2008. Accessed January 12, 2014. [DOI] [PubMed]
- 36.Barnett MJ, Frank J, Wehring H, et al. Analysis of pharmacist-provided medication therapy management (MTM) services in community pharmacies over 7 years. J Manag Care Pharm. 2009;15(1):18–31. doi: 10.18553/jmcp.2009.15.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice, The Clinician’s Guide. 2nd ed. New York, NY: McGraw-Hill; 2004. An overview of pharmaceutical care practice; pp. 1–23. [Google Scholar]
- 38.Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175–1185. doi: 10.1592/phco.23.10.1175.32760. [DOI] [PubMed] [Google Scholar]
- 39.Gable KN, Stunson MJ. Clinical pharmacist interventions on an assertive community treatment team. Community Ment Health J. 2010;46(4):351–355. doi: 10.1007/s10597-009-9252-1. [DOI] [PubMed] [Google Scholar]
- 40.Moore JM, Shartle D, Faudskar L, et al. Impact of a patient-centered pharmacy program and intervention in a high-risk group. J Manag Care Pharm. 2013;19(3):228–236. doi: 10.18553/jmcp.2013.19.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birtcher K. Clinical evaluation of the adult patient. In: Wiggins B, Saseen J, Spinler S, editors. Pharmacist’s Guide to Lipid Management. Lenexa, KS: American College of Clinical Pharmacy; 2008. pp. 20–29. [Google Scholar]
- 42.Rosendorff C, Black HR, Cannon CP, et al. American Heart Association Council on Epidemiology and Prevention. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115(21):2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 43.Jellinger PS, Smith DA, Mehta AE, et al. AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18(suppl 1):1–78. doi: 10.4158/ep.18.s1.1. [DOI] [PubMed] [Google Scholar]
- 44.Diabetes management guidelines. National Diabetes Education Initiative website. http://www.ndei.org/ADA-2013-Guidelines-Diabetes-Hypertension.aspx. Accessed January 12, 2014.
- 45.Schultz N, Albers T, Hammill-Zimmer P, et al. Medication management systems, Inc. http://www.medsmanagement.com/. Accessed February 10, 2014.
- 46.Medication Therapy Management Services (MTMS) Minnesota Department for Human Services website. http://www.dhs.state.mn.us/main/idcplg?IdcService=GET_DYNAMIC_CONVERSION&RevisionSelectionMethod=LatestReleased&dDocName=dhs16_136889. Updated May 1, 2014. Accessed January 12, 2014.
- 47.Omron HEM-790ITCAN. http://www.omronhealthcare.ca/products/hem-790itcan/. Accessed October 1, 2014.
- 48.Health o meter Professional. Eye Level Digital Scale 200KL. http://www.homscales.com/products/eye-level-digital-scale. Accessed October 1, 2014.
- 49.Dale RA, Jensen LH, Krantz MJ. Comparison of two point-of-care lipid analyzers for use in global cardiovascular risk assessments. Ann Pharmacother. 2008;42(5):633–639. doi: 10.1345/aph.1K688. [DOI] [PubMed] [Google Scholar]
- 50.Carter AW. An analysis of the assessment of glycated hemoglobin using A1cNow+ point-of-care device compared to central laboratory testing: an important addition to pharmacist-managed diabetes programs? J Diabetes Sci Tech. 2008;2(5):828–830. doi: 10.1177/193229680800200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrendale JR, Cherian SE, Zineh I, et al. Assessment of glycated hemoglobin using A1CNow+ point-of-care device as compared to central laboratory testing. J Diabetes Sci Tech. 2008;2(5):822–827. doi: 10.1177/193229680800200512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cziraky M, Spinler S. Metabolic syndrome. In: Wiggins B, Saseen J, Spinler S, editors. Pharmacist’s Guide to Lipid Management. Lenexa, KS: American College of Clinical Pharmacy; 2008. pp. 42–59. [Google Scholar]
- 53.Sundvall J, Laatikainen T, Hakala S, et al. Systematic error of serum triglyceride measurements during three decades and the effect of fasting on serum triglycerides in population studies. Clin Chim Acta. 2008;397(1–2):55–59. doi: 10.1016/j.cca.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 54.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 55.Anderson TJ, Grégoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 56.Shoemaker SJ, Hassol A. Understanding the landscape of MTM programs for Medicare, part D: results from a study for the Centers for Medicare & Medicaid services. J Am Pharm Assoc (2003) 2011;51(4):520–526. doi: 10.1331/JAPhA.2011.10210. [DOI] [PubMed] [Google Scholar]
- 57.Kucukarslan SN, Hagan AM, Shimp LA, et al. Integrating medication therapy management in the primary care medical home: a review of randomized controlled trials. Am J Health Syst Pharm. 2011;68(4):335–345. doi: 10.2146/ajhp100405. [DOI] [PubMed] [Google Scholar]
- 58.Nkansah N, Mostovetsky O, Yu C, et al. Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010;(7):CD000336. doi: 10.1002/14651858.CD000336.pub2. 10.1002/14651858.CD000336.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]