Abstract
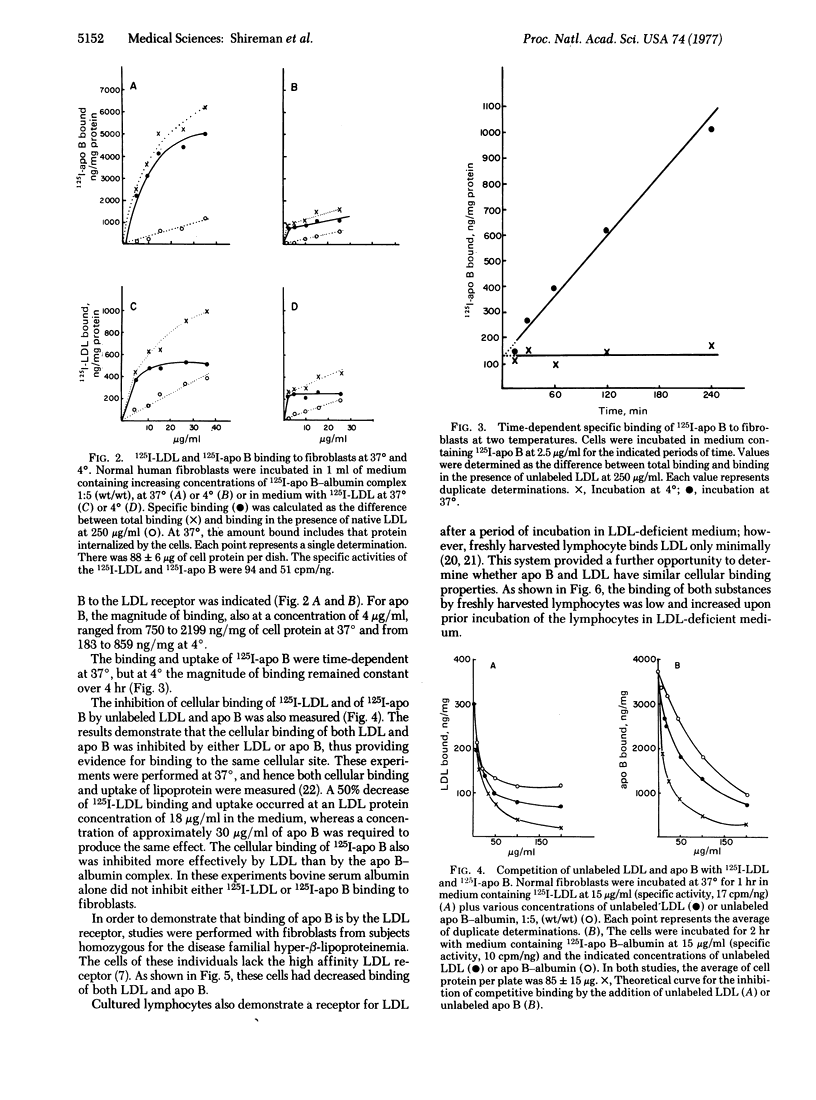
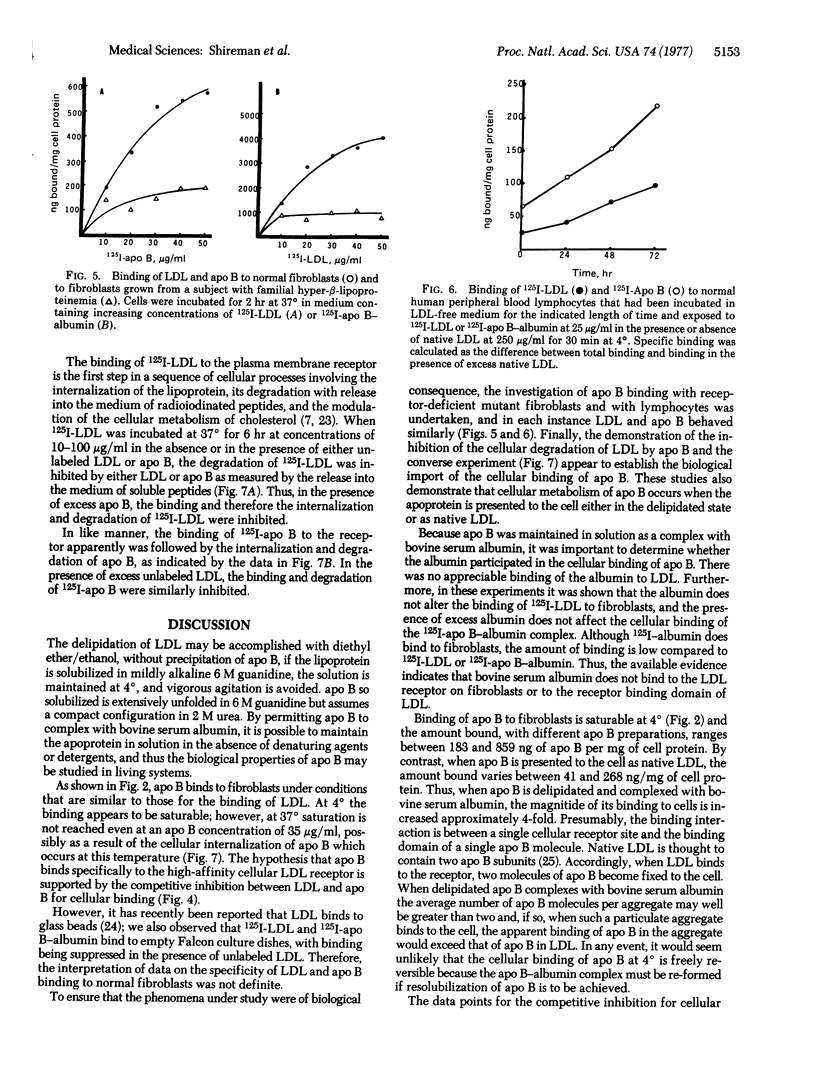
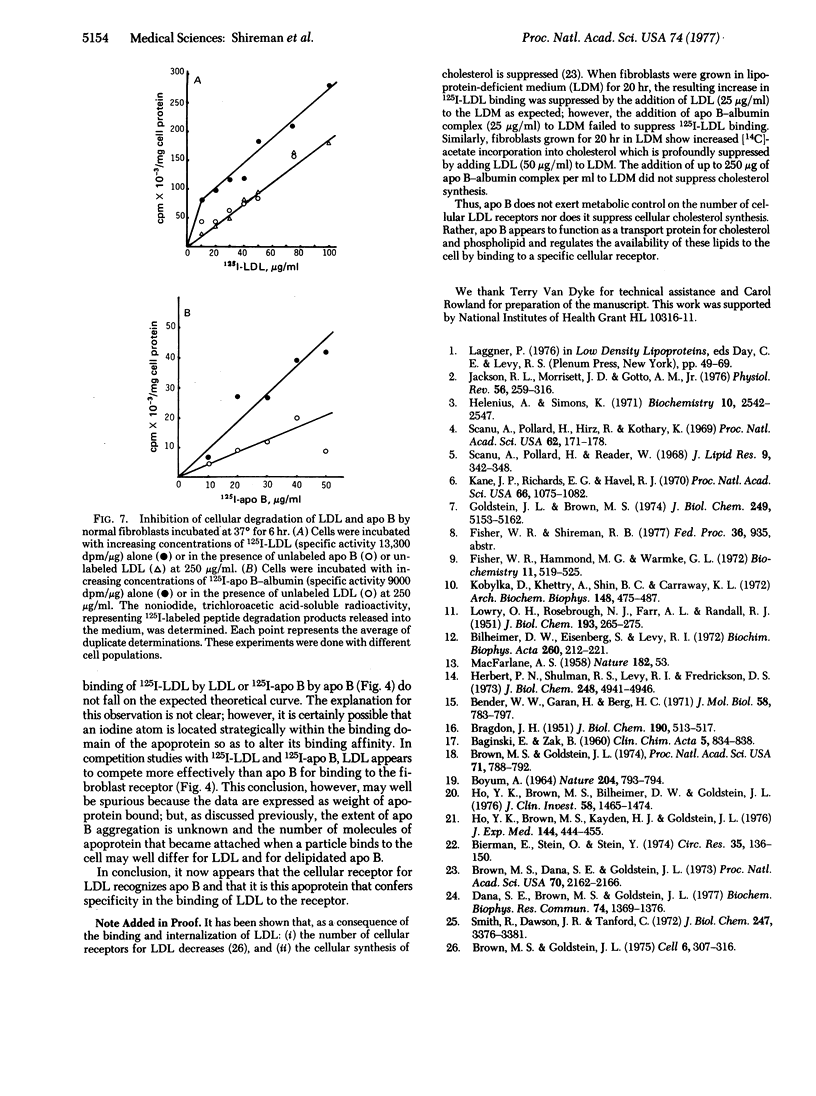
Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) bind specifically to a receptor on fibroblasts, and it has been postulated that the apoprotein of LDL (apo B) confers the specificity of cellular binding. This hypothesis has been tested in the present study with a watersoluble apo B-bovine serum albumin complex. The binding of 125I-labeled apo B to cultured fibroblasts was temperature-dependent. Specific binding ranged between 183 and 859 ng/mg of cell protein at a concentration of 5 μg/ml; at 37°, 750-2199 ng/mg was bound and internalized. The binding of apo B greatly exceeded the amount of 125I-labeled LDL bound at 4° and 37° in the same experiment. Fibroblasts from a subject homozygous for hyper-β-lipoproteinemia showed minimal binding of 125I-labeled LDL, consistent with the absence of the cellular LDL receptor. Such cells also had depressed binding of 125I-labeled apo B.
Lymphocytes grown in lipoprotein-deficient medium demonstrated specific binding of LDL; however, freshly isolated lymphocytes did not show such binding. The binding of 125I-labeled apo B to lymphocytes paralleled the binding of 125I-labeled LDL. Unlabeled LDL and apo B-albumin complex both competitively inhibited the binding of 125I-labeled apo B and 125I-labeled LDL to fibroblasts. When labeled LDL was incubated with fibroblasts for 6 hr at 37°, it underwent cellular internalization and degradation, as measured by the release of 125I-labeled fragments into the medium. This degradation was inhibited by unlabeled apo B. Conversely, 125I-labeled apo B also was internalized and degraded by fibroblasts, and this process was inhibited by LDL. These findings demonstrate that apo B binds specifically to the LDL receptor and that the cellular binding of LDL is determined by this apoprotein.
Keywords: fibroblast, lymphocyte, hyper-β-lipoproteinemia, LDL degradation, apolipoprotein B-bovine serum albumin complex
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAGINSKI E., ZAK B. Micro-determination of serum phosphate and phospholipids. Clin Chim Acta. 1960 Nov;5:834–838. doi: 10.1016/0009-8981(60)90117-0. [DOI] [PubMed] [Google Scholar]
- BOYUM A. SEPARATION OF WHITE BLOOD CELLS. Nature. 1964 Nov 21;204:793–794. doi: 10.1038/204793a0. [DOI] [PubMed] [Google Scholar]
- BRAGDON J. H. Colorimetric determination of blood lipides. J Biol Chem. 1951 Jun;190(2):513–517. [PubMed] [Google Scholar]
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Dana S. E., Brown M. S., Goldstein J. L. Specific, saturable, and high affinity binding of 125I-low density lipoprotein to glass beads. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1369–1376. doi: 10.1016/0006-291x(77)90593-9. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Hammond M. G., Warmke G. L. Measurements of the molecular weight variability of plasma low density lipoproteins among normals and subjects with hyper- -lipoproteinemia. Demonstration of macromolecular heterogeneity. Biochemistry. 1972 Feb 15;11(4):519–525. doi: 10.1021/bi00754a006. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Helenius A., Simons K. Removal of lipids from human plasma low-density lipoprotein by detergents. Biochemistry. 1971 Jun 22;10(13):2542–2547. doi: 10.1021/bi00789a019. [DOI] [PubMed] [Google Scholar]
- Herbert P. N., Shulman R. S., Levy R. I., Fredrickson D. S. Fractionation of the C-apoproteins from human plasma very low density lipoproteins. J Biol Chem. 1973 Jul 25;248(14):4941–4946. [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Kayden H. J., Goldstein J. L. Binding, internalization, and hydrolysis of low density lipoprotein in long-term lymphoid cell lines from a normal subject and a patient with homozygous familial hypercholesterolemia. J Exp Med. 1976 Aug 1;144(2):444–455. doi: 10.1084/jem.144.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Richards E. G., Havel R. J. Subunit heterogeneity in human serum beta lipoprotein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1075–1082. doi: 10.1073/pnas.66.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylka D., Khettry A., Shin B. C., Carraway K. L. Proteins and glycoproteins of the erythrocyte membrane. Arch Biochem Biophys. 1972 Feb;148(2):475–487. doi: 10.1016/0003-9861(72)90166-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Scanu A., Pollard H., Hirz R., Kothary K. On the conformational instability of human serum low-density lipoprotein: effect of temperature. Proc Natl Acad Sci U S A. 1969 Jan;62(1):171–178. doi: 10.1073/pnas.62.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A., Pollard H., Reader W. Properties of human serum low density lipoproteins after modification by succinic anhydride. J Lipid Res. 1968 May;9(3):342–349. [PubMed] [Google Scholar]
- Smith R., Dawson J. R., Tanford C. The size and number of polypeptide chains in human serum low density lipoprotein. J Biol Chem. 1972 Jun 10;247(11):3376–3381. [PubMed] [Google Scholar]