Significance
Purgatorius has been considered a plausible ancestor for primates since it was discovered, but this fossil mammal has been known only from teeth and jaw fragments. We attribute to Purgatorius the first (to our knowledge) nondental remains (ankle bones) which were discovered in the same ∼65-million-year-old deposits as dentitions of this putative primate. This attribution is based mainly on size and unique anatomical specializations known among living euarchontan mammals (primates, treeshrews, colugos) and fossil plesiadapiforms. Results of phylogenetic analyses that incorporate new data from these fossils support Purgatorius as the geologically oldest known primate. These recently discovered tarsals have specialized features for mobility and provide the oldest fossil evidence that suggests arboreality played a key role in earliest primate evolution.
Keywords: Euarchonta, Primates, Paleocene, paleontology, evolution
Abstract
Earliest Paleocene Purgatorius often is regarded as the geologically oldest primate, but it has been known only from fossilized dentitions since it was first described half a century ago. The dentition of Purgatorius is more primitive than those of all known living and fossil primates, leading some researchers to suggest that it lies near the ancestry of all other primates; however, others have questioned its affinities to primates or even to placental mammals. Here we report the first (to our knowledge) nondental remains (tarsal bones) attributed to Purgatorius from the same earliest Paleocene deposits that have yielded numerous fossil dentitions of this poorly known mammal. Three independent phylogenetic analyses that incorporate new data from these fossils support primate affinities of Purgatorius among euarchontan mammals (primates, treeshrews, and colugos). Astragali and calcanei attributed to Purgatorius indicate a mobile ankle typical of arboreal euarchontan mammals generally and of Paleocene and Eocene plesiadapiforms specifically and provide the earliest fossil evidence of arboreality in primates and other euarchontan mammals. Postcranial specializations for arboreality in the earliest primates likely played a key role in the evolutionary success of this mammalian radiation in the Paleocene.
Evidence from the fossil record suggests that placental mammals diversified following the Cretaceous–Paleogene (K–Pg) boundary ∼66 Mya (1, 2). Among the oldest known placental mammals, the putative primate Purgatorius has been documented in the western interior of North America during the first million years after the K–Pg boundary (2–5) to within the first few hundred thousand years of the Paleocene (6). Although the fossil record of Purgatorius has been restricted to dentitions long recognized as uniquely similar to those of primates (3, 4), these anatomical data are limited. Some researchers who have preferred to restrict the order Primates to the crown-clade (i.e., Euprimates) have also questioned the primate affinities of Purgatorius and other Paleogene plesiadapiforms (reputed stem primates) (e.g., refs 7 and 8). Furthermore, several recent phylogenetic analyses have not supported Purgatorius in Primates (9) or even in Placentalia (crown-clade eutherians) (10–12). New evidence supporting Purgatorius as the oldest plesiadapiform primate is derived from tarsal bones collected at the late Puercan (Pu3; ∼65 Mya) Garbani Channel fauna localities in Garfield County, northeastern Montana. Four decades of fieldwork have resulted in the recovery of hundreds of Purgatorius teeth and fragmentary jaws (13). Here we attribute tarsals (astragali and calcanei) to Purgatorius based on size and diagnostic euarchontan and plesiadapiform features (Fig. 1 and SI Appendix). This taxonomic attribution is supported further by the absence of other euarchontan taxa from the Garbani Channel fauna.
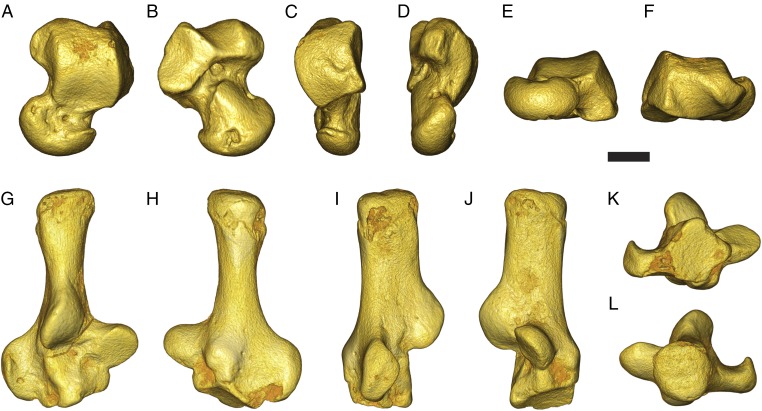
Fig. 1.
Micro-CT scan images of tarsal bones attributed to Purgatorius from the late Puercan (Pu3; ∼65 Mya) Garbani Channel fauna localities in northeastern Montana. UCMP 197509, left astragalus, and UCMP 197517, right calcaneus, are shown in dorsal (A and G), plantar (B and H), lateral (C and I), medial (D and J), distal (E and K), and proximal (F and L) views, respectively. (Scale bar, 1 mm.) See SI Appendix for institutional abbreviations.
Phylogenetic Analysis
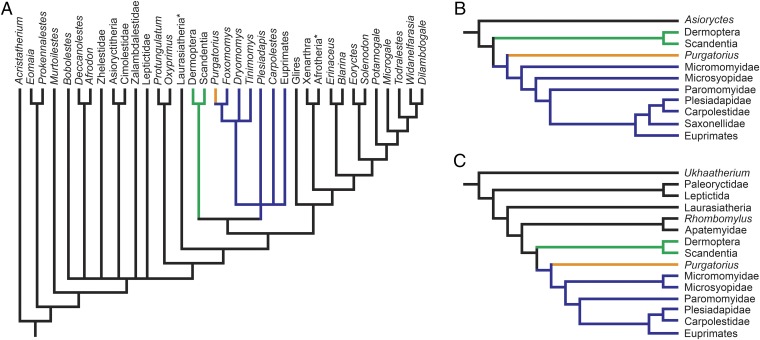
Results from recent broad cladistic analyses that focused on relationships among eutherian mammals do not support primate affinities of Purgatorius and instead place Purgatorius in a clade directly outside Placentalia with the contemporary condylarths (archaic ungulates) Protungulatum and Oxyprimus (10–12). However, the addition of new tarsal data for Purgatorius and increased taxon sampling, including a colugo and four plesiadapiforms, using this same matrix, results in a strict consensus tree that supports a monophyletic Euarchonta with Sundatheria (treeshrews and colugos) as the sister group to a fairly unresolved Primates clade that includes Purgatorius (Fig. 2A). This result is driven mainly by the addition of euarchontan taxa rather than by new character data for Purgatorius, which strongly suggests that the previous support for Purgatorius outside Placentalia and Euarchonta is primarily an artifact of taxon sampling (10, 14). To address this issue further, we included the new Purgatorius tarsal data in two additional analyses that were designed to evaluate relationships within Euarchonta (15) or more broadly within Euarchontoglires (16). Results from both analyses support Purgatorius as the most basal primate (Fig. 2 B and C).
Fig. 2.
Hypotheses of evolutionary relationships of Purgatorius and other eutherian mammals. (A) Simplified resulting strict consensus cladogram based on data modified from ref. 12, Purgatorius tarsals, and five additional euarchontan taxa (colugo Cynocephalus, the micromomyid plesiadapiforms Foxomomys, Dryomomys, and Tinimomys, and the carpolestid plesiadapiform Carpolestes). Asterisks indicate results from a previously published analysis with Laurasiatheria excluding Eulipotyphla and Afrotheria excluding Afrosoricida (12). (B) Simplified resulting single-most-parsimonious cladogram based on data from ref. 15 and Purgatorius tarsals. (C) Simplified resulting strict consensus cladogram based on data from ref. 16 and Purgatorius tarsals. In all cladograms, Sundatheria is supported and indicated in green, Primates is supported and indicated in violet, and Purgatorius is supported as a primate and indicated in orange. See SI Appendix for methods, full tree topologies, and support values.
Description and Comparison of Tarsal Bones
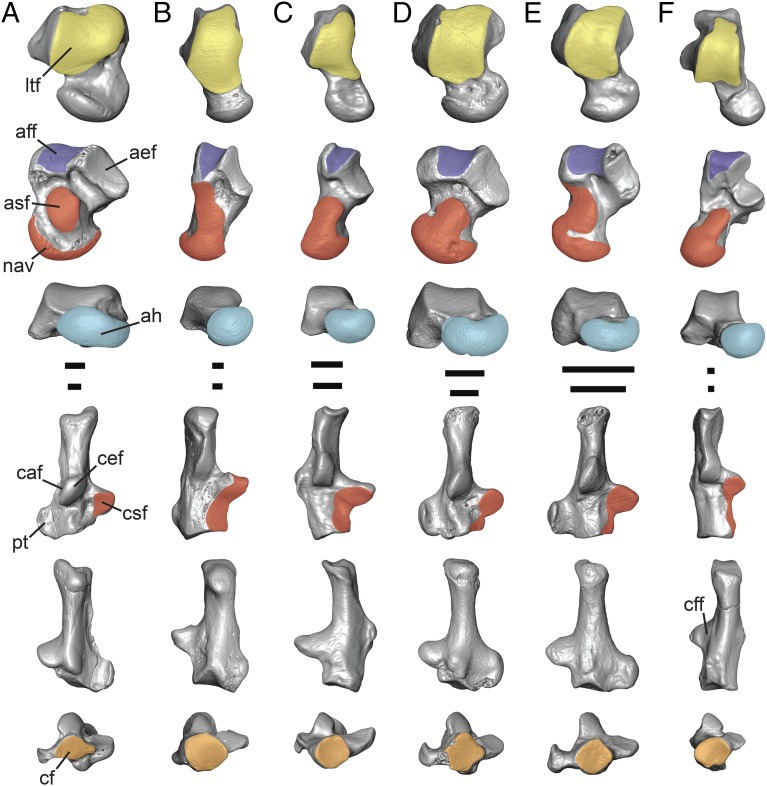
Although previously published cladistic analyses support a close relationship between Purgatorius and the condylarth Protungulatum (10–12), the tarsals attributed to Purgatorius differ considerably from those of Protungulatum by having many characteristics of euarchontan mammals that relate to arboreality (Fig. 3). As in other euarchontans, the upper ankle joint of Purgatorius is more mobile than that of Protungulatum, which has a contact between the fibula and calcaneus that restricts medial–lateral movements at this joint (17). The astragalar trochlea (lateral tibial facet) of Purgatorius is relatively longer than that of Protungulatum, allowing a greater range of dorsi- and plantarflexion (Fig. 3). The trochlea of Purgatorius is medially sloping, is aligned oblique to the long axis of the astragalus, and extends slightly onto the dorsal surface of the astragalar neck, as is consistent with mammals whose feet abduct during dorsiflexion for climbing on vertical supports (18). The lower ankle joint of Purgatorius also is considerably more mobile than that of Protungulatum, especially in having increased capacity for movements between the sustentacular facets of the astragalus and calcaneus. Purgatorius has a saddle-shaped astragalar ectal facet that articulates with and rotates along a longer, moderately proximodistally aligned calcaneal ectal facet (Fig. 1). This morphology suggests a pronounced capacity for inversion and eversion of the foot, which is supported further by the presence of a well-developed distal calcaneal sustentacular facet and a distally extensive astragalar sustentacular facet that contacts the navicular facet (Fig. 3). These distal articular regions would have come into close contact only during strong inversion of the foot. Such movements are facilitated further at the transverse tarsal joint of Purgatorius by the rounded, concave, gliding articulation of the calcaneocuboid facet and its fairly transverse orientation and by the pronounced, rounded navicular facet on the medial side of the astragalar head (Fig. 1). In contrast, Protungulatum has a more ovoid, asymmetrical calcaneocuboid facet that is oriented more obliquely to the long axis of the calcaneus and a less pronounced medial side of the astragalar head, suggesting that Protungulatum had less capacity for pedal inversion and used level-oriented foot positions for locomotion on a flat substrate, as do terrestrial quadrupeds (Fig. 3) (17).
Fig. 3.
Comparison of micro-CT scan images of tarsal bones. Columns illustrate tarsals of condylarth Protungulatum (A), colugo Cynocephalus (B), treeshrew Ptilocercus (C), Purgatorius (D), the micromomyid plesiadapiform Dryomomys (E), and the adapoid euprimate Notharctus (F) right astragali (rows 1–3) and calcanei (rows 4–6) in dorsal (rows 1 and 4), plantar (rows 2 and 5), and distal (rows 3 and 6) views, respectively. Some elements are reversed for clarity. (Scale bars: 1 mm.) aef, astragalar ectal facet; aff, astragalar groove for tendon of musculus flexor fibularis (violet); ah, astragalar head (blue); asf, astragalar sustentacular facet (red); caf, calcaneal fibular facet; cef, calcaneal ectal facet; cf, calcaneocuboid facet (orange); cff, calcaneal groove for tendon of musculus flexor fibularis; csf, calcaneal sustentacular facet (red); ltf, lateral tibial facet (yellow); nav, astragalonavicular facet (red); pt, peroneal tubercle. See SI Appendix for specimen numbers.
Among euarchontans, the tarsals attributed to Purgatorius are uniquely similar to those of other plesiadapiforms in having an astragalus with a medially sloping trochlea and a relatively broad head and a calcaneus with a large peroneal tubercle (Figs. 3 and 4 and SI Appendix) (18). However, it should be noted that currently euarchontan tarsal comparisons outside Primates are limited to the presumably more derived morphologies of extant colugos and treeshrews, given the paucity of postcranial fossils representing these clades. Unlike the level astragalar trochlea of colugos and the most basally divergent treeshrew Ptilocercus, the medially sloping trochlea of Purgatorius may have reduced the potential for lateral sheer of the tibia on the astragalus when inverted foot postures were used during locomotion on large-diameter supports (Fig. 3). The astragalar head of Purgatorius and other plesiadapiforms is broad and ovoid, suggesting frequent use of inverted and everted postures. The large medial aspect of the astragalonavicular facet of Purgatorius likely reflects forces frequently transmitted on the medial side of the head during habitual pedal inversion (17), but the more spherical head of colugos, Ptilocercus, and many euprimates indicates even greater emphasis on inverted postures in these taxa (Fig. 3). Purgatorius also differs from colugos, Ptilocercus, and euprimates in having a calcaneus with a much larger and more laterally projecting peroneal tubercle (Fig. 3), which provides more leverage for tendons of peroneal muscles that contribute to eversion (musculus peroneus longus) and abduction (musculus peroneus brevis) and counterbalance forces that invert the foot (19). The smaller peroneal tubercle in other euarchontans suggests less emphasis on the peroneal muscles for eversion movements and rotational stability, possibly as a mechanical consequence of the greater degree of distal calcaneal elongation present in these taxa (18, 20).
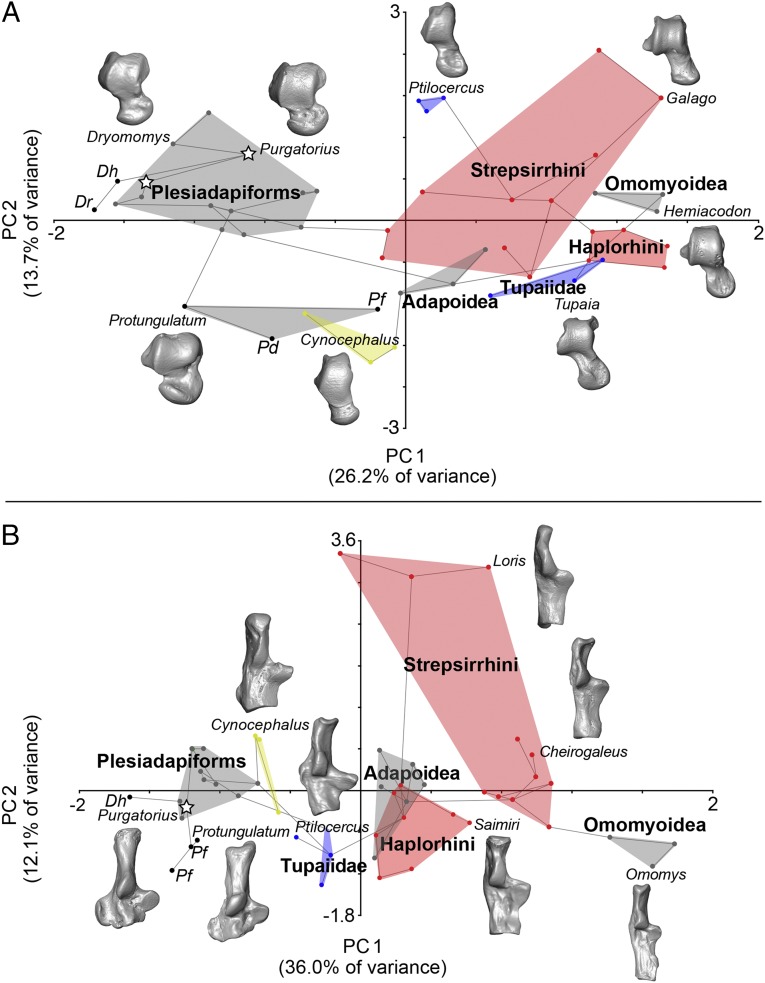
Fig. 4.
Results of principal component analysis of 23 astragalar measurements for 34 species (A) and 25 calcaneal measurements for 33 species (B) (SI Appendix). Lines connecting data points reflect a minimum-spanning tree computed from a Euclidean distance matrix. Polygons encompass taxa including living strepsirrhine and haplorhine euprimates (red), treeshrews (blue), and colugos (yellow). Gray polygons encompass fossil groups including adapoid and omomyoid euprimates, plesiadapiforms, and earliest Paleocene mammals Protungulatum donnae (Pd) and Procerberus formicarum (Pf). Results support tarsals attributed to Purgatorius (starred) as a plesiadapiform, which, like Protungulatum and Cretaceous Deccanolestes (D. hislopi, Dh; D. robustus, Dr), has tarsal features that may be plesiomorphic, such as a large calcaneal peroneal tubercle. See SI Appendix for eigenvalue, percentage variance, and variable component loadings for each principal component.
Micromomyids are the most primitive plesiadapiforms known from skeletons (Fig. 2 B and C) and have been reconstructed as being most similar to Ptilocercus among extant mammals (15). Thus it is significant that Purgatorius, which has teeth very similar to those of primitive micromomyids (21, 22), also shares with that group unique tarsal features including a slightly grooved astragalar trochlea with a relatively high medial ridge and a fairly consistent mediolateral width (Fig. 3), whereas other stem primates have a flat and more medially sloping trochlea that is widest distally. Purgatorius and micromomyids also are most similar in tarsal features related to the tendon of musculus flexor (digitorum) fibularis, which contributes to digital flexion and plantarflexion of the foot and is important for pedal grasping. These taxa have a very large and mediolaterally wide flexor fibularis groove on the astragalus (Fig. 3), as is consistent with the large origination areas indicating sizeable flexor muscles on the tibia and fibula of micromomyids (23). However, the corresponding groove for the tendon of flexor fibularis on the plantar aspect of the calcaneal sustentaculum is shallow in Purgatorius and micromomyids, as it is in treeshrews and colugos (Fig. 3). The presence of a deep flexor fibularis groove on the calcaneal sustentaculum has been considered a synapomorphy for primates related to stronger hallucal grasping, whereas this muscle has been considered to play a less active role in treeshrews and colugos (18). In fact, a deep flexor fibularis groove is present on the calcaneus of euprimates and more derived plesiadapiforms (including paromomyids and plesiadapoids). The combination of a large groove for the tendon of the flexor fibularis on the astragalus and absence of a deep groove on the calcaneus in Purgatorius and micromomyids may be a primitive retention in these taxa. Similar characteristics are present in Protungulatum and the Cretaceous eutherian Deccanolestes, whose affinities lie well outside the Euarchonta (Fig. 2).
Discussion
The evolution of diagnostic euprimate traits associated with grasping, leaping, and an enhanced visual system has long been thought to relate in part to arboreality (24), although substrate preferences of our earliest primate ancestors have been less clear. Certain features of euprimates, such as grasping hands and feet, already had evolved to various degrees among plesiadapiforms (15, 20, 23, 25–28). Nearly all plesiadapiform species are known only from fossil dentitions, and the several known partial skeletons belong to fairly derived and relatively late-occurring members of their respective clades (23). Based on ecological inferences from the shape of the skull and teeth, it has been suggested that arboreality and herbivory evolved independently in plesiadapiforms and euprimates following their divergence from a ground-dwelling, insectivorous ancestor (29). However, tarsals of Purgatorius reported here indicate instead that arboreality was characteristic of the oldest and most primitive known stem primate. Purgatorius is more primitive than other plesiadapiforms and euprimates in retaining three lower incisors, four lower premolars, and molars with taller trigonids and more acute cusps that likely reflect an omnivorous diet that included a large proportion of insects (3, 13). Like the dentition (3, 4), the tarsals of Purgatorius reflect a plesiomorphic state that is sufficiently primitive to have given rise to the more derived morphologies present in all later primates.
The major radiation of angiosperms in the Late Cretaceous continued throughout the earliest Paleocene and dominated megafloras in the North American western interior (30, 31). Within this context, the immigration of Purgatorius represents the infusion of a unique arboreal mammal into North America during the first million years following the K–Pg boundary (32). Increased size of seeds and fruits is correlated with increases in the proportions of animal-dispersed taxa during this time (33) and would have provided an arboreal and omnivorous primate such as Purgatorius with angiosperm products including fruits, flowers, and associated insect pollinators (15, 34–36). Therefore, the postcranial specializations for arboreality documented in Purgatorius would have allowed this animal to access resources that were not directly available to many contemporary terrestrial mammals, such as Protungulatum. The fossil record provides a direct test to evaluate adaptive scenarios, however incremental (8), and future recovery and analysis of early euarchontan fossils will continue to improve our understanding of primate origins. The previously unidentified fossils of Purgatorius described here suggest that the divergence of primates from other mammals was not a dramatic event. Instead, the beginning of primate evolutionary history likely involved subtle changes in the postcranial skeleton that allowed easier navigation and improved access to food resources in an arboreal setting.
Materials and Methods
Regression Analysis.
To assess whether tarsals described here (SI Appendix, Table S1) are of a size consistent with their attribution to the Purgatorius dental sample from the Garbani Channel fauna (SI Appendix, Fig. S1), least squares linear regression analyses were run in Microsoft Excel to evaluate the scaling relationship between the natural log area of the second lower molar and astragalar tibial trochlea width, as well as between the natural log second lower molar area and calcaneal cuboid facet area for euarchontan mammals. Skeletal elements from a sample of 60 dentally associated skeletons of euarchontans including extant taxa and fossil plesiadapiforms were microCT scanned, and digital reconstructions were measured using Avizo 6 software (SI Appendix, Table S2). The 95% confidence limits on the prediction interval of tooth size from postcranial element dimensions were generated using equation 17.29 of ref. 37. Dimensions from isolated tarsals and teeth of Purgatorius (SI Appendix, Table S3) then were plotted on the resulting regression equations (SI Appendix, Fig. S2).
Principal Component Analysis.
To evaluate our qualitative observations that the tarsals attributed to Purgatorius are generally similar to those of euarchontan mammals and are specifically similar to those of plesiadapiforms, we ran principal component analysis on the correlation matrix derived from 18 linear and 5 angular astragalar measurements (SI Appendix, Fig. S3A) following ref. 38 for 48 individuals representing 34 species (SI Appendix, Table S4) and 19 linear and 6 angular calcaneal measurements (SI Appendix, Fig. S3B) following ref. 39 for 54 individuals representing 33 species (SI Appendix, Table S5). Additionally, we ran a cluster analysis using the correlation matrix as our similarity metric and using the paired group method for linking cases. All analyses were run using PAST v. 2.16 (40). All linear measurements were size-standardized using the geometric mean of a subset of the measures. Angular measurements are reported in degrees but were analyzed in radians. The expanded taxonomic sample includes Puercan mammals, fossil plesiadapiforms and euprimates, and extant euarchontans (SI Appendix, Tables S4 and S5). All tarsals were microCT scanned, and digital reconstructions were created and measured using Avizo 6 software. Eigenvalue, percentage variance, and variable component loadings were recorded for each principal component (SI Appendix, Tables S6 and S7).
Phylogenetic Analysis.
Cladistic analysis using maximum parsimony was performed in TNT (41) on three revised character matrices (12, 15, 16). Four plesiadapiforms, one colugo, and new Purgatorius tarsal data were added to the character matrix of ref. 12, and new Purgatorius tarsal data were added to the character matrices of refs. 15 and 16. In all analyses, New Technology Search was used to obtain the stabilized consensus five times, and resulting most parsimonious trees (MPTs) were used as starting trees in a Traditional Heuristic Search that was carried out using tree bisection reconnection (TBR). All resulting MPTs were used to obtain a strict consensus, and, following the methods of ref. 12, the Pruned Trees function was used to identify the least stable taxa, which were removed using the Prune Taxa function if large polytomies were present. The Tree Filter function was used to delete longer trees and duplicate MPTs. Bremer branch supports were calculated using the Traditional Search option (10 replicates per run with TBR enforced) from 50,000 suboptimal trees up to 10 steps longer than the most parsimonious tree. See SI Appendix for more detailed methodology, list of specimens analyzed, and specific modifications to character matrices (SI Appendix, Tables S8–S10).
Supplementary Material
Acknowledgments
We thank P. Holroyd, E. Sargis, C. Manz, G. Wilson, L. Debey, K. Pugh, C. Sprain, P. Renne, W. Mitchell III, M. Silcox, and A. Hill for helpful discussions; eight anonymous reviewers and the editor for helpful comments; the Engdahl family and Bureau of Land Management for help with fieldwork; J. VanHouten, S. Judex, and C. Ruben for CT scanning assistance; and G. Yapuncich, A. Garberg, J. Butler, and J. Lovoi for segmenting CT scans. S.G.B.C. was supported by National Science Foundation (NSF) Grant SBE-1028505 (to E. J. Sargis and S.G.B.C.), the Leakey Foundation, and a Brooklyn College Tow Faculty Travel Fellowship. J.I.B. was supported by NSF Grant SBR-9616194 (to G. F. Gunnell, P. D. Gingerich, and J.I.B.) and Yale Institute for Biospheric Studies. D.M.B. was supported by NSF Grant BCS 1317525 (to E. Seiffert and D.M.B.). W.A.C. was supported by NSF Grant EAR 9505847. This work also was supported by the Doris O. and Samuel P. Welles Research Fund, University of California Museum of Paleontology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421707112/-/DCSupplemental.
References
- 1.O’Leary MA, et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 2013;339(6120):662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- 2.Renne PR, et al. Time scales of critical events around the Cretaceous-Paleogene boundary. Science. 2013;339(6120):684–687. doi: 10.1126/science.1230492. [DOI] [PubMed] [Google Scholar]
- 3.Van Valen L, Sloan RE. The earliest primates. Science. 1965;150(3697):743–745. doi: 10.1126/science.150.3697.743. [DOI] [PubMed] [Google Scholar]
- 4.Szalay FS, Delson E. Evolutionary History of the Primates. Academic; New York, NY: 1979. [Google Scholar]
- 5.Clemens WA. Purgatorius, an early paromomyid primate (Mammalia) Science. 1974;184(4139):903–905. doi: 10.1126/science.184.4139.903. [DOI] [PubMed] [Google Scholar]
- 6.Fox RC, Scott CS. A new, early Puercan (earliest Paleocene) species of Purgatorius (Plesiadapiformes, Primates) from Saskatchewan, Canada. J Paleontol. 2011;85(3):537–548. [Google Scholar]
- 7.Martin RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton Univ Press; Princeton, NJ: 1990. [Google Scholar]
- 8.Cartmill M. Primate origins, human origins, and the end of higher taxa. Evol Anthropol. 2012;21(6):208–220. doi: 10.1002/evan.21324. [DOI] [PubMed] [Google Scholar]
- 9.Ni X, et al. The oldest known primate skeleton and early haplorhine evolution. Nature. 2013;498(7452):60–64. doi: 10.1038/nature12200. [DOI] [PubMed] [Google Scholar]
- 10.Wible JR, Rougier GW, Novacek MJ, Asher RJ. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 2007;447(7147):1003–1006. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]
- 11.Wible JR, Rougier GW, Novacek MJ, Asher RJ. The eutherian mammal Maelestes gobiensis from the Late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bull Am Mus Nat Hist. 2009;327:1–123. [Google Scholar]
- 12.Goswami A, et al. A radiation of arboreal basal eutherian mammals beginning in the Late Cretaceous of India. Proc Natl Acad Sci USA. 2011;108(39):16333–16338. doi: 10.1073/pnas.1108723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens WA. Purgatorius (Plesiadapiformes, Primates? Mammalia), a Paleocene immigrant into northeastern Montana: Stratigraphic occurrences and incisor proportions. Bull Carnegie Mus Nat Hist. 2004;36:3–13. [Google Scholar]
- 14.Boyer DM, et al. New postcrania of Deccanolestes from the Late Cretaceous of India and their bearing on the evolutionary and biogeographic history of euarchontan mammals. Naturwissenschaften. 2010;97(4):365–377. doi: 10.1007/s00114-010-0648-0. [DOI] [PubMed] [Google Scholar]
- 15.Bloch JI, Silcox MT, Boyer DM, Sargis EJ. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc Natl Acad Sci USA. 2007;104(4):1159–1164. doi: 10.1073/pnas.0610579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silcox MT, Bloch JI, Boyer DM, Houde P. Cranial anatomy of Paleocene and Eocene Labidolemur kayi (Mammalia: Apatotheria), and the relationships of the Apatemyidae to other mammals. Zool J Linn Soc. 2010;160(4):773–825. [Google Scholar]
- 17.Szalay FS, Decker RL. Origins, evolution, and function of the tarsus in Late Cretaceous Eutheria and Paleocene primates. In: Jenkins FA Jr, editor. Primate Locomotion. Academic; New York: 1974. pp. 223–254. [Google Scholar]
- 18.Szalay FS, Drawhorn G. Evolution and diversification of the Archonta in an arboreal milieu. In: Luckett WP, editor. Comparative Biology and Evolutionary Relationships of Tree Shrews. Plenum; New York: 1980. pp. 133–169. [Google Scholar]
- 19.Boyer DM, Patel BA, Larson SG, Stern JT., Jr Telemetered electromyography of peroneus longus in Varecia variegata and Eulemur rubriventer: Implications for the functional significance of a large peroneal process. J Hum Evol. 2007;53(2):119–134. doi: 10.1016/j.jhevol.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Boyer DM, Seiffert ER, Gladman JT, Bloch JI. Evolution and allometry of calcaneal elongation in living and extinct primates. PLoS ONE. 2013;8(7):e67792. doi: 10.1371/journal.pone.0067792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox RC. The dentition and relationship of the Paleocene primate Micromomys Szalay, with description of a new species. Can J Earth Sci. 1984;21(11):1262–1267. [Google Scholar]
- 22.Chester SGB, Bloch JI. Systematics of Paleogene Micromomyidae (Euarchonta, Primates) from North America. J Hum Evol. 2013;65(2):109–142. doi: 10.1016/j.jhevol.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Bloch JI, Boyer DM. New skeletons of Paleocene-Eocene Plesiadapiformes: A diversity of arboreal positional behaviors in early primates. In: Ravosa MJ, Dagosto M, editors. Primate Origins: Adaptations and Evolution. Plenum; New York: 2007. pp. 535–582. [Google Scholar]
- 24.Smith GE. The Evolution of Man. Smithsonian Institution Annual Report. Smithsonian Institution; Washington, DC: 1912. [Google Scholar]
- 25.Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298(5598):1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- 26.Sargis EJ, Boyer DM, Bloch JI, Silcox MT. Evolution of pedal grasping in Primates. J Hum Evol. 2007;53(1):103–107. doi: 10.1016/j.jhevol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Kirk EC, Lemelin P, Hamrick MW, Boyer DM, Bloch JI. Intrinsic hand proportions of euarchontans and other mammals: Implications for the locomotor behavior of plesiadapiforms. J Hum Evol. 2008;55(2):278–299. doi: 10.1016/j.jhevol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Boyer DM, Yapuncich GS, Chester SGB, Bloch JI, Godinot M. Hands of early primates. Am J Phys Anthropol. 2013;152(Suppl 57):33–78. doi: 10.1002/ajpa.22392. [DOI] [PubMed] [Google Scholar]
- 29.Kay RF, Cartmill M. Cranial morphology and adaptations of Palaechthon nacimienti and other Paromomyidae (Plesiadapoidea? Primates), with a description of a new genus and species. J Hum Evol. 1977;6(1):19–53. [Google Scholar]
- 30.Wilf P, Johnson KR, Huber BT. Correlated terrestrial and marine evidence for global climate changes before mass extinction at the Cretaceous-Paleogene boundary. Proc Natl Acad Sci USA. 2003;100(2):599–604. doi: 10.1073/pnas.0234701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arens NC, Allen SE. A florule from the base of the Hell Creek Formation in the type area of eastern Montana: Implications for vegetation and climate. Geo Soc Amer Special Paper. 2014;503:173–207. [Google Scholar]
- 32.Clemens WA. Evolution of the mammalian fauna across the Cretaceous-Tertiary boundary in northeastern Montana and other areas of the western interior. Geo Soc Amer Special Paper. 2002;361:217–245. [Google Scholar]
- 33.Eriksson O, Friis EM, Löfgren P. Seed size, fruit size, and dispersal systems in angiosperms from the early Cretaceous to the late Tertiary. Am Nat. 2000;156(1):47–58. doi: 10.1086/303367. [DOI] [PubMed] [Google Scholar]
- 34.Szalay FS. The beginnings of primates. Evolution. 1968;22(1):19–36. doi: 10.1111/j.1558-5646.1968.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 35.Sussman RW, Tab Rasmussen D, Raven PH. Rethinking primate origins again. Am J Primatol. 2013;75(2):95–106. doi: 10.1002/ajp.22096. [DOI] [PubMed] [Google Scholar]
- 36.Wing SL, Tiffney BH. The reciprocal interaction of angiosperm evolution and tetrapod herbivory. Rev Palaeobot Palynol. 1987;50(1-2):179–210. [Google Scholar]
- 37.Zar JH. Biostatistical Analysis. Prentice-Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]
- 38.Boyer DM, Seiffert ER, Simons EL. Astragalar morphology of Afradapis, a large adapiform primate from the earliest late Eocene of Egypt. Am J Phys Anthropol. 2010;143(3):383–402. doi: 10.1002/ajpa.21328. [DOI] [PubMed] [Google Scholar]
- 39.Boyer DM. New Cranial and Postcranial Remains of Late Paleocene Plesiadapidae (“Plesiadapiforms,” Mammalia) from North America and Europe: Description and Evolutionary Implications. Stony Brook University; Stony Brook, NY: 2009. [Google Scholar]
- 40.Hammer O, Harper DAT, Ryan PD. Past: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4(1):1–9. [Google Scholar]
- 41.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24(5):774–786. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.