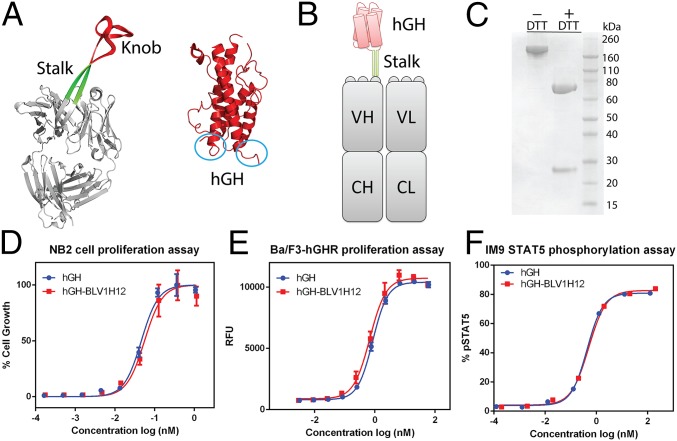
Fig. 1.
(A) X-ray crystal structures of bovine antibody BLV1H12 Fab fragment [Protein Data Bank (PDB) ID code 4K3D] and human growth hormone (PDB ID code 1HGU). The N and C termini of the four-helix–bundle protein are circled in blue. (B) A schematic representation of hGH–BLV1H12 fusion. The knob domain of BLV1H12 is replaced by hGH. (C) SDS/PAGE analysis of hGH–BLV1H12 fusion. (D) hGH–BLV1H12 fusion protein stimulates proliferation of rat NB2-11 cells in a dose-dependent manner. Cells were treated with various concentrations of hGH and hGH–BLV1H12 fusion proteins and cell viability was quantified using PrestoBlue reagent; assays were performed in triplicate. (E) hGH-dependent Ba/F3-hGHR cells proliferate in a dose-dependent manner upon treatment with hGH–BLV1H12. Cells were treated with various concentrations of hGH and hGH–BLV1H12 fusion proteins, and cell viability was quantified using PrestoBlue reagent; assays were performed in triplicate. RFU, relative fluorescence units. (F) hGH–BLV1H12 fusion protein stimulates STAT5 phosphorylation in IM9 cells. Serum-starved IM9 cells were treated with various concentrations of hGH and hGH–BLV1H12 for 10 min, and phosphorylation of STAT5 was quantified by flow cytometry analysis. Assays were performed in duplicate. Error bars represent the standard deviation.