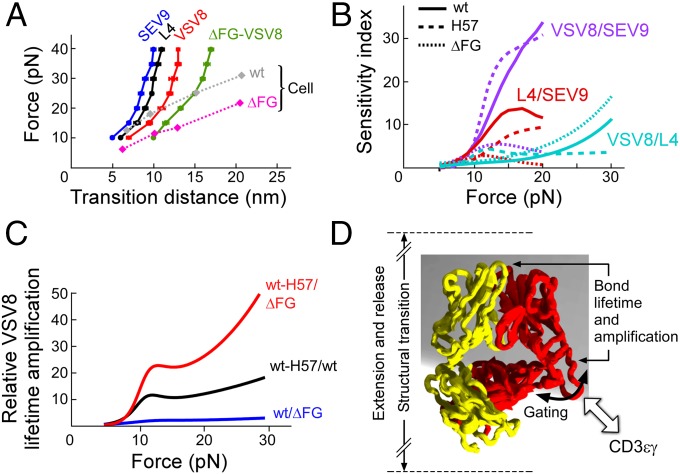
Fig. 4.
Force-dependent structural transitions, ligand sensitivity, amplification factors and an αβTCR model. (A) The major structural transition distance, measured as the difference in bead position before and after the transition, is plotted vs. force for WT-VSV8 cognate peptide (red), -L4 partial agonist (black), -SEV9 irrelevant ligand (blue), and ΔFG-VSV8 (green) showing a ligand dependence with greater ligand potency exhibiting larger structural transition distances in SM systems. Force-transition distance plots for cell systems; SMSC (diamonds with dotted lines) exhibit greater displacement with VSV8 for ΔFG than for WT and are generally larger than the SM transitions. (B) Sensitivity plots comparing lifetime ratios of various antigens for WT (solid), H57 (dashed), and ΔFG (dotted). Plots were constructed from fits in Fig. 2. (C) Amplification factors associated with lifetime enhancement for VSV8 binding including ratios of WT-H57/ΔFG (red), WT-H57/WT (black), and WT/ΔFG (blue). Amplification factors rise abruptly at 10 pN, are greatest with stabilization of the FG loop, and increase generally as a function of force. (D) Model for force-induced motions and gating associated with the CβFG loop region. The FG loop region couples to the binding interface strength and conformational change magnitude. Associations with molecules such as CD3εγ may dramatically stabilize the CβFG loop, influencing bond lifetime and force transfer response of the loaded αβTCR–pMHC complex system.