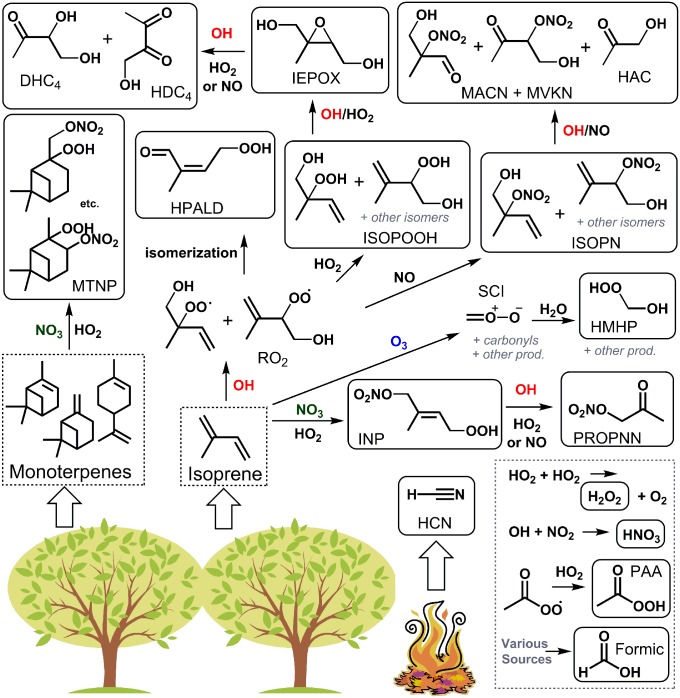
Fig. 1.
Formation pathways for the oxidation products of isoprene and monoterpenes included in this study. Thick arrows indicate primary emission. Representative isomers and reaction pathways are shown. Flux data are reported for compounds shown in solid boxes. In HO2-rich environments, the OH-initiated reaction of isoprene generates ISOPOOH, which are subsequently oxidized to epoxydiols (IEPOX) in an OH-conserving mechanism with almost quantitative yields (88). The photooxidation of IEPOX produces C4 dihydroxy carbonyls (DHC4), C4 hydroxy dicarbonyls (HDC4), HAC, formic acid, and other products (42, 89). In NO-rich environments, the minor route of the OH-initiated reaction of isoprene produces ISOPN, which are subsequently oxidized to HAC, hydroxy nitrates with backbones of MACN and MVKN, and other products (54, 90, 91). The major route of the NO-dominated OH-initiated reaction (not shown) produces carbonyls such as methacrolein, methyl vinyl ketone, and HAC, among other products. PROPNN is formed primarily from the oxidation-induced fragmentation of larger nitrates. A temperature-dependent isomerization of the isoprene RO2 generates the HPALD (56, 92). The O3-initated oxidation of isoprene or other exocyclic compounds forms the C1 stabilized Criegee intermediate (SCI), among other products, which reacts with water to produce HMHP and formic acid (43). NO3-initated oxidation of isoprene and monoterpenes, followed by the HO2 reaction, produces INP and MTNP, respectively (93). PAA is thought to predominantly form through the HO2 reaction with acetylperoxy radical [CH3C(O)OO], which has several anthropogenic and biogenic sources (94). The main source of HCN is biomass burning (52). Formic acid has many sources, including direct emission from canopies, photooxidation at surfaces and in the gas phase, ozonolysis, and secondary chemistry (6).