What are Geobacillus?
The genus Geobacillus includes thermophilic Gram-positive spore-forming bacteria that form a phylogenetically coherent clade within the family Bacillaceae. They are of great interest for biotechnology (as discussed below). These thermophiles seem to be ubiquitous; viable Geobacillus spores can be isolated in large quantities not only from hot environments such as hydrothermal vents, but also, paradoxically, from cool soils and cold ocean sediments (Zeigler, 2005).
These bacteria were previously categorized as ‘Group 5’ within the genus Bacillus but were subsequently split into the new genus Geobacillus (Nazina et al., 2001). Many Geobacillus strains were previously described as belonging to a single species Bacillus stearothermophilus, but it was clear that there was great heterogeneity in physiology, preferred temperature range and other phenotypic characteristics among these strains. For example, see Fig. 1 showing three distinct colony morphologies among three strains described as ‘B. stearothermophilus’. It is now absolutely clear that there are several distinct species within Geobacillus and these can be distinguished by both genotype and phenotype (Nazina et al., 2001; Banat et al., 2004; Zeigler, 2005; Dinsdale et al., 2011; Coorevits et al., 2012).
Fig 1.
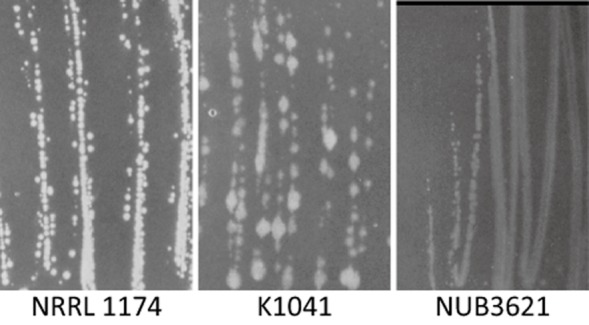
Diverse colony morphologies of strains classified as ‘G. stearothermophilus’. Strains NRRL 1174, K1041 and NUB3621 were streaked-out on tryptic soy broth plates and incubated overnight at 50°C. Plates were photographed under identical conditions.
Why are Geobacillus species of interest for biotechnology?
Geobacillus spp. are of interest for biotechnology as source of thermostable enzymes and natural products, digesters of lignocellulose, bioremediators of hydrocarbons, producers of bio-fuel, cellular factories for heterologous expression of enzymes and as hosts for directed evolution (Wiegel et al., 1985; Niehaus et al., 1999; Couñago and Shamoo, 2005; Marchant et al., 2006; Cripps et al., 2009; Taylor et al., 2009; Tabachnikov and Shoham, 2013). Industrially important enzymes originating from Geobacillus spp. include lipases (Schmidt-Dannert et al., 1998), glycoside hydrolases (Fridjonsson et al., 1999; Bartosiak-Jentys et al., 2013; Suzuki et al., 2013), N-acylhomoserine lactonase (Seo et al., 2011) and DNA polymerase I (Sandalli et al., 2009) and protease (Chen et al., 2004) among others. The advantages of using thermophilic bacteria as whole-cell biocatalysts were recently discussed in this journal (Taylor et al., 2011) and include reduced risk of contamination, acceleration of biochemical processes and easier maintenance of anaerobic conditions. These bacteria also tend to ferment a wide range of substrates, utilizing both cellobiose and pentose sugars. In the context of bioethanol production, there is the additional advantage of reduced cooling costs and easier removal and recovery of the volatile product by sparging or partial vacuum thus also avoiding ethanol poisoning of the bacteria (Taylor et al., 2009). Less positively, Geobacillus spp. are common contaminants in the dairy and food industries (Burgess et al., 2010).
Which genomes have been sequenced?
At the time of writing (28 July 2014), 29 Geobacillus genome sequences are available (Table 1). These include representatives of all the major phylogenetic groups within the genus and include representatives of the species G. thermoleovorans, G. kaustophilus, G. thermocatenulatus, G. thermodenitrificans, G. stearothermophilus, G. caloxylosilyticus and G. thermoglucosidans (formerly G. thermoglucosidasius) as well as several strains that have not been assigned to named species (Fig. 2). Genome sequences are also available for some other thermophilic members of the Bacillaceae, such as Paenibacillus lautus (Mead et al., 2012) and Bacillus coagulans (Xu et al., 2013) and for Geobacillus-infecting bacteriophage (Marks and Hamilton, 2014), but these will not be discussed here. The team who sequenced the genome of Geobacillus sp. MAS1 described this strain as ‘G. thermopakistaniensis’, but this is not a validly named species and no justification was provided for its proposal as a new species (Siddiqui et al., 2014). On the basis of its recN sequence, a useful phylogenetic marker for Geobacillus spp. (Zeigler, 2005), strain MAS1 is closely related to the type strains of G. kaustophilus and G. thermoleovorans (Fig. 2). Strain NUB3621 was described as ‘G. stearothermophilus’ but as has been previously noted (Studholme et al., 1999; Zeigler, 2005; Blanchard et al., 2014), this strain is phylogenetically distinct from B. stearothermophilus sensu strictu and is more closely related to G. caldoxylsilyticus and, to a lesser extent, G. thermoglucosidans (Fig. 2). For more than half of the sequenced genomes, papers have been published describing and/or announcing the sequence data and usually indicating the particular features of the strain that motivated its sequencing. An insightful discussion of the biological lessons from Geobacillus genomes was previously published earlier this year, including surveys of genes involved in breakdown of plant-derived lignocellulose (Zeigler, 2005); but at that time, only 10 genome sequences were available.
Table 1.
Geobacillus strains whose genomes have been sequenced as of 26 July 2014
| Species and strain | Motivation for sequencing | Accession number | References |
|---|---|---|---|
| G. caldoxylosilyticus CIC9 | Not known | NZ_AMRO01000000.1 | n. a. |
| G. caldoxylosilyticus NBRC 107762 | Not known | BAWO01000000.1 | n. a. |
| G. kaustophilus GBlys | Lysogenic, containing an integrated prophage | NZ_BASG01000001.1 | (Doi et al., 2013) |
| G. kaustophilus HTA426 | Source of novel glycoside hydrolases (6-phospho-β-glycosidase and β-fucosidase) | NC_006510.1 | (Takami et al., 2004) |
| G. sp. A8 | Not known | NZ_AUXP01000001.1 | n. a. |
| G. sp. C56-T3 | Not known | NC_014206.1 | n. a. |
| G. sp. CAMR12739 | Hemicellulose degradation | JHUR01000001.1 | (De Maayer et al., 2014) |
| G. sp. CAMR5420 | Hemicellulose degradation | JHUS01000001.1 | (De Maayer et al., 2014) |
| G. sp. FW23 | Potential for degradation and utilization of oil (bioremediation of oil spills) | JGCJ01000001.1 | (Pore et al., 2014) |
| G. sp. G11MC16 | Not known | NZ_ABVH01000001.1 | n. a. |
| G. sp. GHH01 | Source if thermostable and thermo-active secreted lipase | NC_020210.1 | (Wiegand et al., 2013) |
| G. sp. JF8 | Degrades biphenyl and polychlorinated biphenyls (PCB) | NC_022080.4 | (Shintani et al., 2014) |
| G. sp. MAS1 | Potential source of useful enzyme-encoding genes | NZ_AYSF01000001.1 | (Siddiqui et al., 2014) |
| G. sp. WCH70 | Not known | NC_012793.1 | n. a. |
| G. sp. WSUCF1 | Abel to grow on lignocellulosic substrates | NZ_ATCO01000001.1 | (Bhalla et al., 2013) |
| G. sp. Y4.1MC1 | Not known | NC_014650.1 | n. a. |
| G. sp. Y412MC52 | Not known | NC_014915.1 | n. a. |
| G. sp. Y412MC61 | Not known | NC_013411.1 | n. a. |
| G. stearothermophilus ATCC 7953 | Not known | JALS01000001.1 | n. a. |
| G. stearothermophilus NUB3621 | Genetically amenable host strain for metabolic engineering | AOTZ01000001.1 | (Blanchard et al., 2014) |
| G. thermocatenulatus GS-1 | Not known | JFHZ01000001.1 | n. a. |
| G. thermodenitrificans NG80-2 | Denitrification and degradation of long-chain alkanes, facilitating oil recovery in oil reservoirs | NC_009328.1 | (Feng et al., 2007) |
| G. thermodenitrificans subsp. thermodenitrificans DSM 465 | Comparative genomics between the alkane-utilizing NG80-2 and this strain which is unable to utilize alkanes | NZ_AYKT01000001.1 | (Yao et al., 2013) |
| G. thermoglucosidans TNO-09.020 | Contaminant in dairy-processing environment | NZ_CM001483.1 | (Zhao et al., 2012) |
| G. thermoglucosidasius C56-YS93 | Not known | NC_015660.1 | n. a. |
| G. thermoglucosidasius NBRC 107763 | Not known | BAWP01000001.1 | n. a. |
| G. thermoleovorans B23 DNA | Alkane degrader with unidentified alkane monooxygenase | BATY01000001.1 | (Boonmak et al., 2013) |
| G. thermoleovorans CCB_US3_UF5 | Not known | NC_016593.1 | (Muhd Sakaff et al., 2012) |
Names are given as found in the GenBank sequence database. n.a., not available.
Fig 2.
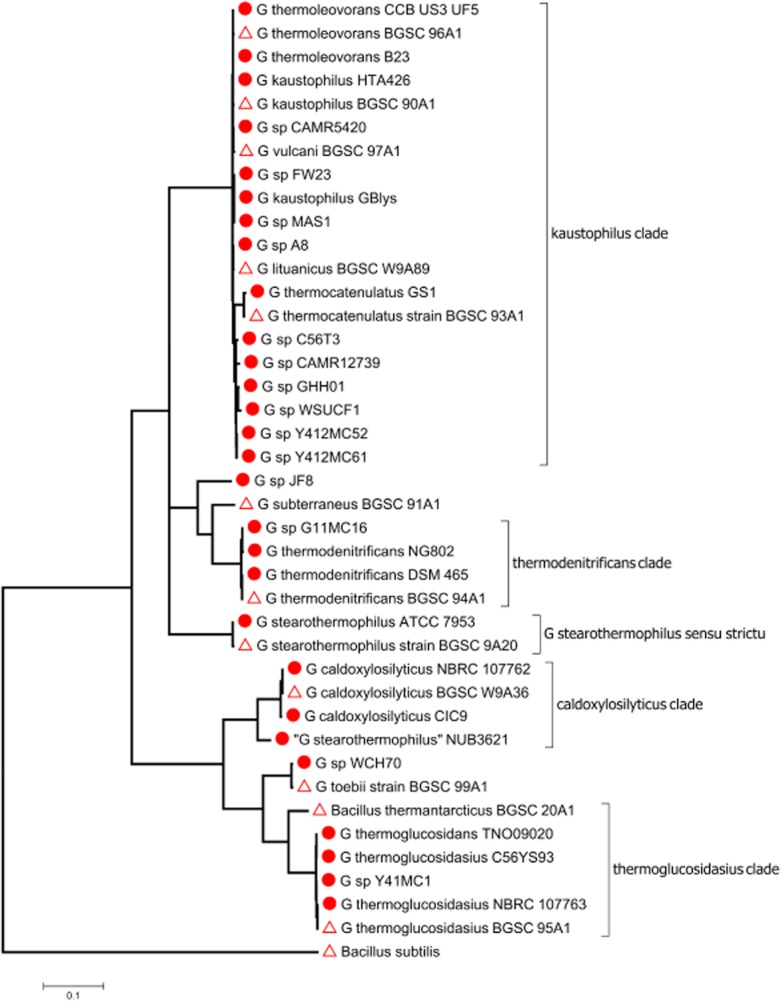
Phylogenetic relationships among sequenced strains of Geobacillus inferred from a multiple sequence alignment of recN sequences. The circles indicate strains whose genomes have been sequenced, as listed in Table 1. The triangles indicate type strains of the various Geobacillus species; recN sequences from these are taken from a previous phylogenetic analysis by Zeigler (2005). The maximum-likelihood tree was generated using mega6 (Tamura et al., 2013).
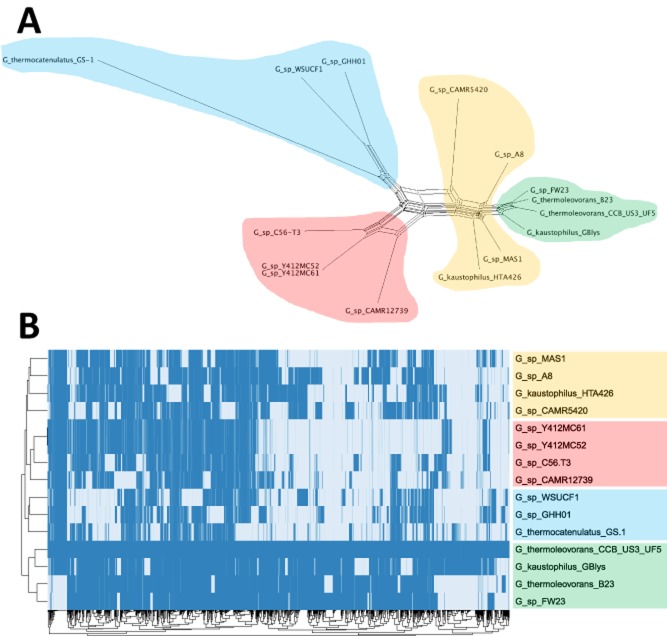
The phylogenetic group within Geobacillus most richly represented by genome sequences is the clade containing G. thermoleovorans, G. kaustophilus and G. thermocatenulatus (see the ‘kaustophilus clade’ in Fig. 2). Based solely of sequences of the recN phylogenetic marker, it is not possible to precisely resolve relationships among sequenced strains within this group (Fig. 2). However, the availability of complete genome sequence data enables phylogenetic analysis based on single-nucleotide variants over the entire core genome, offering much greater resolution (Fig. 3A). According to the core-genome-wide phylogenetic analysis, the two strains assigned as G. kaustophilus do not form a phylogenetically coherent monophyletic clade. On the other hand, the two strains of G. thermoleovorans are closely related and share 99.4% nucleotide sequence identity [based on mummer2 alignments (Delcher et al., 2002)]. Strain FW23 also appears to fall within this clade and, subject to phenotypic characterization, can probably be considered a member of this species too. Geobacillus thermocatenulatus GS-1 is much more divergent, sharing only 94% to 95% identity with the other strains in the clade, which is consistent with the recN-based analysis (Fig. 2). Strains Y412MC52 and YP412MC61 appear to be extremely closely related to each other, sharing 99.8% sequence identity and showing no detectable differences in gene content. Nucleotide sequence identities between clades are much lower; between G. kaustophilus and G. thermoglucosidans, there is approximately 84% identity.
Fig 3.
Relationships among sequenced genomes within the G. kaustophilus clade resolved using whole-genome sequence data. The phylogenetic network in panel A was based on a concatenation of 1722 variant single-nucleotide sites in 1 874 967 nucleotides of the core genome present in all 15 genomes. The network was generated using the neighbornetalgorithm (Bryant and Moulton, 2004) implemented in the splitstree software package (Huson, 1998). The heat-map in B indicates the presence (dark blue) and absence (light blue) of each of 931 non-core genes from the genome of G. thermoleovorans CCB US3 UF6 across the same 15 genomes appearing in A. The gene-content clusters are shaded in the same colours in both panels. The heat-map was rendered using Raivo Kolde's pheatmap package in R (R Development Core Team, R, 2013).
The considerable amount of reticulation in the phylogenetic network (Fig. 3A) suggests significant horizontal genetic transfer within and among these species. This is further illustrated by the extent of variation in the variable component of the genome (Fig. 3B). Out of 3887 genes on the chromosome of G. thermoleovorans CCB US3 UF5, a total of 931 (approximately 24%) are variable (that is, they are absent from at least one of the other sequenced genomes). The global pattern of gene content (Fig. 3B) broadly reflects the phylogenetic relationships (Fig. 3A): according to gene content, the genomes fall into four main clusters, indicated by four different colours of shading in Fig. 3B, which correspond to four zones of the phylogenetic network, shaded with the same colours in Fig. 3A. However, there are numerous genes whose distribution across the genomes is incongruent with core-genome phylogeny, again suggesting extensive horizontal transfer.
What benefits has the sequencing of Geobacillus genomes brought?
The availability of complete Geobacillus genome sequences has enabled or accelerated the discovery, cloning and exploitation of natural products. For example, the availability of the NG80-2 genome sequence (Feng et al., 2007) enabled the discovery of thermostable homologues of the lantibiotic nisin in G. thermodenitrificans (Begley et al., 2009; Garg et al., 2012), opening the possibility of replacing nisin as a food preservative and veterinary antibiotic with more-stable alternatives. Lantibiotics appear to be widely distributed among sequenced Geobacillus species. For example, the genome of G. kaustophilus HTA426 contains two lantibiotic-biosynthesis gene clusters (centred on the genes for YP_146139 and YP_146147) that are both conserved in the recently sequenced Geobacillus sp. CAMR12739. The NG80-2 genome sequence also enabled discovery of the first nitrous oxide reductase gene from a Gram-positive, and a novel thermophilic long-chain alkane monooxygenase (Feng et al., 2007). Furthermore, the genome sequence enabled proteomics-level confirmation of pathways for catabolism of long-chain alkanes (Feng et al., 2007) and aromatics (Li et al., 2012).
Many of the Geobacillus genome sequencing projects reported genes potentially encoding thermostable homologues of useful enzymes. In some cases, the genome sequences have been used to clone and express the genes of interest and characterize the enzyme for biotechnological potential. For example, the genome of G. kaustophilus HTA426 was recently mined for members of the glycoside hydrolase family 1, which have potential uses in synthesizing therapeutic oligosaccharides (Suzuki et al., 2013). The genome sequence of the alkane-utilizing G. thermoleovorans B23 (Boonmak et al., 2013) revealed a cluster of three long-chain alkane monooxygenase genes with homology to that of NG80-2 that showed activity in vivo when heterologously expressed in Pseudomonas fluorescens (Boonmak et al., 2014). Recently, a novel thermostable endo-xylanase was cloned and expressed from Geobacillus sp. WSUCF1 (Bhalla et al., 2014) following the sequencing of its genome (Bhalla et al., 2013).
Genome sequencing has revealed that interesting traits are often encoded on chromosomes rather than on the chromosome. For example, the biphenyl-degrading pathway of Geobacillus sp. JF8 (Mukerjee-Dhar et al., 2005; Shintani et al., 2014) and the long-chain alkane monooxygenase of G. thermodenitrificans NG80-2 (Feng et al., 2007) are both located on plasmids. The dynamic loss and gain of such mobile elements presumably explains, in part, the physiological differences between natural isolates of Geobacillus spp. and it also suggests that these bacteria might be engineered to express new traits by introduction of recombinant plasmids. Indeed, progress has been made in developing plasmid shuttle vectors for heterologous expression in Geobacillus spp. (Thompson et al., 2008; Bartosiak-Jentys et al., 2013).
The value of genome sequencing goes beyond cataloguing potentially useful enzymes, as exemplified by the recently published genomic study of strain NUB3621 (Blanchard et al., 2014). Some previous attempts to fully exploit the potential of Geobacillus strains as whole-cell catalysts have been frustrated by the paucity of genetic and genomic resources (my own PhD research project in the mid-1990s being a case in point; Studholme, 1998). However, strain NUB3621 is a promising laboratory workhorse strain. It is one of the few Geobacillus strains that has been shown to be readily transformable with plasmid DNA (Wu and Welker, 1989); protocols have been developed for genetic analysis (Chen et al., 1986) and a genetic map has been available for more than two decades (Vallier and Welker, 1990). Strain NUB3621 is a mutant derived from wild-type strain NUB36 that lacks its parent strain's restriction-modification system and this probably contributes to transformation efficiency. Incidentally, and consistent with this, we observed that transformation efficiency was significantly affected by the methylation status of the plasmid DNA (Thompson et al., 2008).
Being one of the most genetically amenable Geobacillus strains, NUB3621 was obviously a high priority for genome sequencing. But rather than simply announcing and describing its genome sequence, the authors went on to show how the genome sequence could be exploited to further develop the strain as a host for heterologous expression and metabolic engineering (Blanchard et al., 2014). Specifically, they used the genome sequence to clone two promoters and incorporated them into plasmid vectors: one for inducible gene expression and one constitutive. The authors also mention that they tried other promoters that did not work so well; presumably, the availability of the genome sequence allowed them to relatively quickly screen a number of candidates until they found the best ones. The combination of a genome sequence, allowing relatively facile construction of expression and/or knock-out constructs and a global view of metabolism, along with transformability and a wide range of growth temperatures [between 39 and 75°C (Wu and Welker, 1991)] make NUB3621 a strong candidate as the preferred thermophilic host for rationally designed metabolic engineering.
What's next?
The availability of complete (or nearly complete) genome sequences for nearly 30 Geobacillus strains (Table 1) as well as large-scale proteomic data for at least one (Feng et al., 2007; Li et al., 2012) should certainly accelerate cloning, expression and characterization of novel thermostable and thermo-active enzymes, at least in an academic research context. However, there has been relatively little industrial uptake of enzymes from thermophiles, with much greater use of proteins originating from mesophiles but engineered for thermo-stability (Haki and Rakshit, 2003; Taylor et al., 2011). The convergence of genomic data and transformability, at least for strain NUB3621, should help to remove the barriers to greater exploitation of thermophiles. However, genome sequences are not yet publicly available for the handful of other readily transformable Geobacillus strains such as G. thermodenitrificans K1041 (Narumi et al., 1992), G. stearothermophilus IFO 12550 (Imanaka et al., 1982), NRRL 1174 (Liao et al., 1986) and G. thermoglucosidasius TN (Thompson et al., 2008). Furthermore, although it is possible to predict the metabolic networks of bacteria from complete genome sequence, there is a need for comprehensive testing of these predictions through metabolomics. Only then can we rationally design genetic interventions to predictably manipulate metabolism. And finally, palaeo-genomics of ancient Geobacillus spores, which may be viable after billions of years of dormancy, might shed light on population-genetics and evolutionary processes over timescales that we previously assumed to be intractable (Nicholson, 2003; Zeigler, 2005).
Acknowledgments
The strains K1041, NUB3621 and NRRL1174 shown in Fig. 1 were kindly given by I. Narumi (Japan Atomic Energy Research Institute, Takasaki, Japan), N. Welker (Northwestern University, Evanston, USA) and H. Liao (Cangene, Ontario, Canada) respectively.
Conflict of interest
None declared.
References
- Banat IM, Marchant R. Rahman TJ. Geobacillus debilis sp. nov., a novel obligately thermophilic bacterium isolated from a cool soil environment, and reassignment of Bacillus pallidus to Geobacillus pallidus comb. nov. Int J Syst Evol Microbiol. 2004;54:2197–2201. doi: 10.1099/ijs.0.63231-0. [DOI] [PubMed] [Google Scholar]
- Bartosiak-Jentys J, Hussein AH, Lewis CJ. Leak DJ. Modular system for assessment of glycosyl hydrolase secretion in Geobacillus thermoglucosidasius. Microbiology. 2013;159:1267–1275. doi: 10.1099/mic.0.066332-0. [DOI] [PubMed] [Google Scholar]
- Begley M, Cotter PD, Hill C. Ross RP. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl Environ Microbiol. 2009;75:5451–5460. doi: 10.1128/AEM.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Kainth AS. Sani RK. Draft genome sequence of lignocellulose-degrading thermophilic bacterium Geobacillus sp. strain WSUCF1. Genome Announc. 2013;1 doi: 10.1128/genomeA.00595-13. : pii: e00595-13. doi: 10.1128/genomeA.00595-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Bischoff KM, Uppugundla N, Balan V. Sani RK. Novel thermostable endo-xylanase cloned and expressed from bacterium Geobacillus sp. WSUCF1. Bioresour Technol. 2014;165:314–318. doi: 10.1016/j.biortech.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Blanchard K, Robic S. Matsumura I. Transformable facultative thermophile Geobacillus stearothermophilus NUB3621 as a host strain for metabolic engineering. Appl Microbiol Biotechnol. 2014;98:6715–6723. doi: 10.1007/s00253-014-5746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonmak C, Takahasi Y. Morikawa M. Draft genome sequence of Geobacillus thermoleovorans strain B23. Genome Announc. 2013;1 doi: 10.1128/genomeA.00944-13. : pii: e00944-13. doi: 10.1128/genomeA.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonmak C, Takahashi Y. Morikawa M. Cloning and expression of three ladA-type alkane monooxygenase genes from an extremely thermophilic alkane-degrading bacterium Geobacillus thermoleovorans B23. Extremophiles. 2014;18:515–523. doi: 10.1007/s00792-014-0636-y. [DOI] [PubMed] [Google Scholar]
- Bryant D. Moulton V. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Burgess SA, Lindsay D. Flint SH. Thermophilic bacilli and their importance in dairy processing. Int J Food Microbiol. 2010;144:215–225. doi: 10.1016/j.ijfoodmicro.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Chen X-G, Stabnikova O, Tay J-H, Wang J-Y. Tay ST-L. Thermoactive extracellular proteases of Geobacillus caldoproteolyticus, sp. nov., from sewage sludge. Extremophiles. 2004;8:489–498. doi: 10.1007/s00792-004-0412-5. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Wojcik SF. Welker NE. Genetic analysis of Bacillus stearothermophilus by protoplast fusion. J Bacteriol. 1986;165:994–1001. doi: 10.1128/jb.165.3.994-1001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorevits A, Dinsdale AE, Halket G, Lebbe L, De Vos P, Van Landschoot A. Logan NA. Taxonomic revision of the genus Geobacillus: emendation of GeobacillusG. stearothermophilusG. jurassicusG. toebiiG. thermodenitrificans and G. thermoglucosidans (nom. corrig., formerly ‘thermoglucosidasius’); transfer of Bacillus thermantarcticus. Int J Syst Evol Microbiol. 2012;62:1470–1485. doi: 10.1099/ijs.0.030346-0. [DOI] [PubMed] [Google Scholar]
- Couñago R. Shamoo Y. Gene replacement of adenylate kinase in the gram-positive thermophile Geobacillus stearothermophilus disrupts adenine nucleotide homeostasis and reduces cell viability. Extremophiles. 2005;9:135–144. doi: 10.1007/s00792-004-0428-x. [DOI] [PubMed] [Google Scholar]
- Cripps RE, Eley K, Leak DJ, Rudd B, Taylor M, Todd M, et al. Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab Eng. 2009;11:398–408. doi: 10.1016/j.ymben.2009.08.005. [DOI] [PubMed] [Google Scholar]
- De Maayer P, Williamson CE, Vennard CT, Danson MJ. Cowan DA. Draft genome sequences of Geobacillus sp. strains CAMR5420 and CAMR12739. Genome Announc. 2014;2 doi: 10.1128/genomeA.00567-14. : pii: e00567-14. doi: 10.1128/genomeA.00567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher AL, Phillippy A, Carlton J. Salzberg SL. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 2002;30:2478–2483. doi: 10.1093/nar/30.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale AE, Halket G, Coorevits A, Van Landschoot A, Busse H-J, De Vos P. Logan NA. Emended descriptions of Geobacillus thermoleovorans and Geobacillus thermocatenulatus. Int J Syst Evol Microbiol. 2011;61:1802–1810. doi: 10.1099/ijs.0.025445-0. [DOI] [PubMed] [Google Scholar]
- Doi K, Mori K, Martono H, Nagayoshi Y, Fujino Y, Tashiro K, et al. Draft genome sequence of Geobacillus kaustophilus GBlys, a lysogenic strain with bacteriophage OH2. Genome Announc. 2013;1 doi: 10.1128/genomeA.00634-13. : pii: e00634-13. doi: 10.1128/genomeA.00634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Wang W, Cheng J, Ren Y, Zhao G, Gao C, et al. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc Natl Acad Sci USA. 2007;104:5602–5607. doi: 10.1073/pnas.0609650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridjonsson O, Watzlawick H, Gehweiler A. Mattes R. Thermostable alpha-galactosidase from Bacillus stearothermophilus NUB3621: cloning, sequencing and characterization. FEMS Microbiol Lett. 1999;176:147–153. doi: 10.1111/j.1574-6968.1999.tb13655.x. [DOI] [PubMed] [Google Scholar]
- Garg N, Tang W, Goto Y, Nair SK. van der Donk WA. Lantibiotics from Geobacillus thermodenitrificans. Proc Natl Acad Sci USA. 2012;109:5241–5246. doi: 10.1073/pnas.1116815109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haki GD. Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresour Technol. 2003;89:17–34. doi: 10.1016/s0960-8524(03)00033-6. [DOI] [PubMed] [Google Scholar]
- Huson DH. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Fujii M, Aramori I. Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982;149:824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu J, Wang W, Ding P. Feng L. Proteomics analysis of aromatic catabolic pathways in thermophilic Geobacillus thermodenitrificans NG80-2. J Proteomics. 2012;75:1201–1210. doi: 10.1016/j.jprot.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Liao H, McKenzie T. Hageman R. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci USA. 1986;83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant R, Sharkey FH, Banat IM, Rahman TJ. Perfumo A. The degradation of n-hexadecane in soil by thermophilic geobacilli. FEMS Microbiol Ecol. 2006;56:44–54. doi: 10.1111/j.1574-6941.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- Marks TJ. Hamilton PT. Characterization of a thermophilic bacteriophage of Geobacillus kaustophilus. Arch Virol. 2014 doi: 10.1007/s00705-014-2101-8. . doi: 10.1007/s00705-014-2101-8. [DOI] [PubMed] [Google Scholar]
- Mead DA, Lucas S, Copeland A, Lapidus A, Cheng J-F, Bruce DC, et al. Complete genome sequence of Paenibacillus strain Y4.12MC10, a novel Paenibacillus lautus strain isolated from Obsidian hot spring in Yellowstone National Park. Stand Genomic Sci. 2012;6:381–400. doi: 10.4056/sigs.2605792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhd Sakaff MKL, Abdul Rahman AY, Saito JA, Hou S. Alam M. Complete genome sequence of the thermophilic bacterium Geobacillus thermoleovorans CCB_US3_UF5. J Bacteriol. 2012;194:1239. doi: 10.1128/JB.06580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee-Dhar G, Shimura M, Miyazawa D, Kimbara K. Hatta T. bph genes of the thermophilic PCB degrader, Bacillus sp. JF8: characterization of the divergent ring-hydroxylating dioxygenase and hydrolase genes upstream of the Mn-dependent BphC. Microbiology. 2005;151:4139–4151. doi: 10.1099/mic.0.28437-0. [DOI] [PubMed] [Google Scholar]
- Narumi I, Sawakami K, Nakamoto S, Nakayama N, Yanagisawa T, Takahashi N. Kihara H. A newly isolated Bacillus stearotheromophilus K1041 and its transformation by electroporation. Biotechnol Tech. 1992;6:83–86. [Google Scholar]
- Nazina TN, Tourova TP, Poltaraus AB, Novikova E, V, Grigoryan AA, Ivanova AE, et al. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilusBacillus thermocatenulatusBacillus thermoleovoransBacillus kaustophilusBacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilusG. th. Int J Syst Evol Microbiol. 2001;51:433–446. doi: 10.1099/00207713-51-2-433. [DOI] [PubMed] [Google Scholar]
- Nicholson WL. Using thermal inactivation kinetics to calculate the probability of extreme spore longevity: implications for paleomicrobiology and lithopanspermia. Orig Life Evol Biosph. 2003;33:621–631. doi: 10.1023/a:1025789032195. [DOI] [PubMed] [Google Scholar]
- Niehaus F, Bertoldo C, Kähler M. Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- Pore SD, Arora P. Dhakephalkar PK. Draft genome sequence of Geobacillus sp. strain FW23, isolated from a formation water sample. Genome Announc. 2014;2 doi: 10.1128/genomeA.00352-14. : pii: e00352-14. doi: 10.1128/genomeA.00352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, R. R: a language and environment for statistical computing. R Found Stat Comput. 2013;1:409. [Google Scholar]
- Sandalli C, Singh K, Modak MJ, Ketkar A, Canakci S, Demir I. Belduz AO. A new DNA polymerase I from Geobacillus caldoxylosilyticus TK4: cloning, characterization, and mutational analysis of two aromatic residues. Appl Microbiol Biotechnol. 2009;84:105–117. doi: 10.1007/s00253-009-1962-3. [DOI] [PubMed] [Google Scholar]
- Schmidt-Dannert C, Pleiss J. Schmid RD. A toolbox of recombinant lipases for industrial applications. Ann N Y Acad Sci. 1998;864:14–22. doi: 10.1111/j.1749-6632.1998.tb10284.x. [DOI] [PubMed] [Google Scholar]
- Seo M-J, Lee B-S, Pyun Y-R. Park H. Isolation and characterization of N-acylhomoserine lactonase from the thermophilic bacterium, Geobacillus caldoxylosilyticus YS-8. Biosci Biotechnol Biochem. 2011;75:1789–1795. doi: 10.1271/bbb.110322. [DOI] [PubMed] [Google Scholar]
- Shintani M, Ohtsubo Y, Fukuda K, Hosoyama A, Ohji S, Yamazoe A, et al. Complete genome sequence of the thermophilic polychlorinated biphenyl degrader Geobacillus sp. strain JF8 (NBRC 109937) Genome Announc. 2014;2:e01213–13. doi: 10.1128/genomeA.01213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MA, Rashid N, Ayyampalayam S. Whitman WB. Draft genome sequence of Geobacillus thermopakistaniensis strain MAS1. Genome Announc. 2014;2 doi: 10.1128/genomeA.00559-14. : pii: e00559-14. doi: 10.1128/genomeA.00559-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme DJ. Metabolic engineering of thermophilic Bacillus species for ethanol production. PhD Thesis. London: Deparment of Biochemistry, Imperial College; 1998. [Google Scholar]
- Studholme DJ, Jackson RA. Leak DJ. Phylogenetic analysis of transformable strains of thermophilic Bacillus species. FEMS Microbiol Lett. 1999;172:85–90. doi: 10.1111/j.1574-6968.1999.tb13454.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Okazaki F, Kondo A. Yoshida K. Genome mining and motif modifications of glycoside hydrolase family 1 members encoded by Geobacillus kaustophilus HTA426 provide thermostable 6-phospho-β-glycosidase and β-fucosidase. Appl Microbiol Biotechnol. 2013;97:2929–2938. doi: 10.1007/s00253-012-4168-z. [DOI] [PubMed] [Google Scholar]
- Tabachnikov O. Shoham Y. Functional characterization of the galactan utilization system of Geobacillus stearothermophilus. FEBS J. 2013;280:950–964. doi: 10.1111/febs.12089. [DOI] [PubMed] [Google Scholar]
- Takami H, Takaki Y, Chee G-J, Nishi S, Shimamura S, Suzuki H, et al. Thermoadaptation trait revealed by the genome sequence of thermophilic Geobacillus kaustophilus. Nucleic Acids Res. 2004;32:6292–6303. doi: 10.1093/nar/gkh970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A. Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MP, Eley KL, Martin S, Tuffin MI, Burton SG. Cowan DA. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 2009;27:398–405. doi: 10.1016/j.tibtech.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Taylor MP, Zyl L, van Tuffin IM, Leak DJ. Cowan DA. Genetic tool development underpins recent advances in thermophilic whole-cell biocatalysts. Microb Biotechnol. 2011;4:438–448. doi: 10.1111/j.1751-7915.2010.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AH, Studholme DJ, Green EM. Leak DJ. Heterologous expression of pyruvate decarboxylase in Geobacillus thermoglucosidasius. Biotechnol Lett. 2008;30:1359–1365. doi: 10.1007/s10529-008-9698-1. [DOI] [PubMed] [Google Scholar]
- Vallier H. Welker NE. Genetic map of the Bacillus stearothermophilus NUB36 chromosome. J Bacteriol. 1990;172:793–801. doi: 10.1128/jb.172.2.793-801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand S, Rabausch U, Chow J, Daniel R, Streit WR. Liesegang H. Complete genome sequence of Geobacillus sp. strain GHH01, a thermophilic lipase-secreting bacterium. Genome Announc. 2013;1:e0009213. doi: 10.1128/genomeA.00092-13. . doi: 10.1128/genomeA.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel J, Ljungdahl LG. Demain AL. The importance of thermophilic bacteria in biotechnology. Crit Rev Biotechnol. 1985;3:39–108. [Google Scholar]
- Wu L. Welker NE. Temperature-induced prote in synthesis in Bacillus stearothermophilus NUB36. J Bacteriol. 1991;173:4889–4892. doi: 10.1128/jb.173.15.4889-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ. Welker NE. Protoplast transformation of Bacillus stearothermophilus NUB36 by plasmid DNA. J Gen Microbiol. 1989;135:1315–1324. doi: 10.1099/00221287-135-5-1315. [DOI] [PubMed] [Google Scholar]
- Xu K, Su F, Tao F, Li C, Ni J. Xu P. Genome sequences of two morphologically distinct and thermophilic Bacillus coagulans strains, H-1 and XZL9. Genome Announc. 2013;1:4563–4564. doi: 10.1128/genomeA.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Ren Y. Wang W. Genome sequence of a thermophilic BacillusGeobacillus thermodenitrificans DSM465. Genome Announc. 2013;1 doi: 10.1128/genomeA.01046-13. : pii: e01046-13. doi: 10.1128/genomeA.01046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler DR. Application of a recN sequence similarity analysis to the identification of species within the bacterial genus Geobacillus. Int J Syst Evol Microbiol. 55:1171–1179. doi: 10.1099/ijs.0.63452-0. [DOI] [PubMed] [Google Scholar]
- Zeigler DR. The Geobacillus paradox: why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology. 2014;160:1–11. doi: 10.1099/mic.0.071696-0. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Caspers MP, Abee T, Siezen RJ. Kort R. Complete genome sequence of Geobacillus thermoglucosidans TNO-09.020, a thermophilic sporeformer associated with a dairy-processing environment. J Bacteriol. 2012;194:4118. doi: 10.1128/JB.00318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]