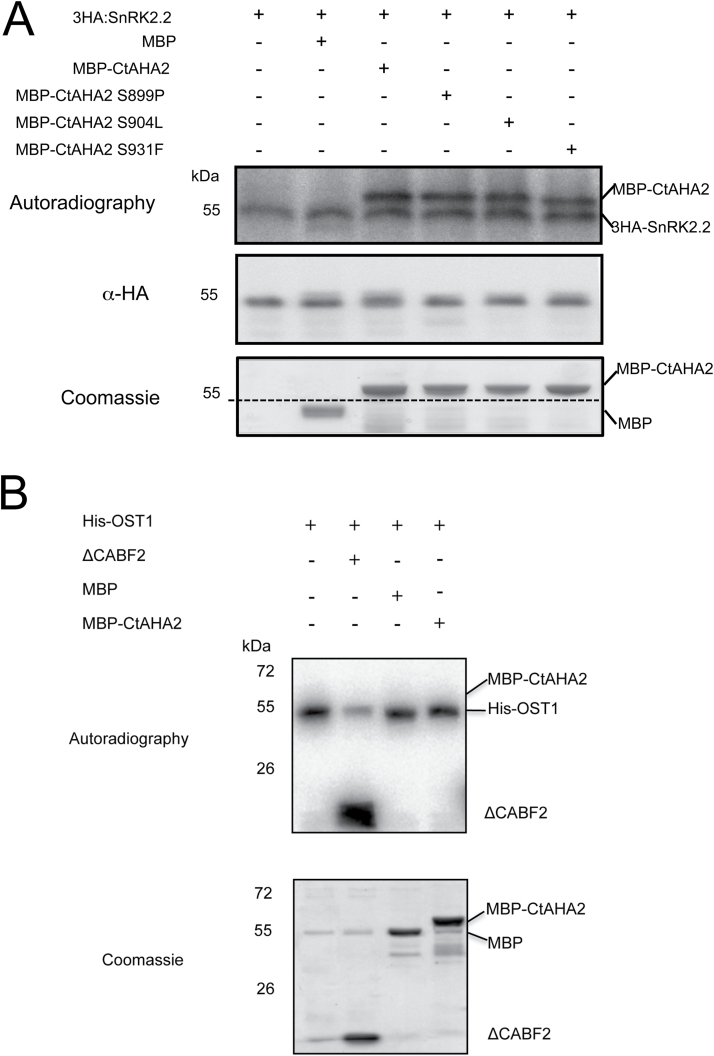
Fig. 4.
SnRK2.2 specifically phosphorylates the C-terminal regulatory domain (Ct) of AHA2 PM H+-ATPase in vitro. (A) 3HA–SnRK2.2, MBP–CtAHA2, and MBP were purified and added to the kinase assay as indicated. Autophosphorylation of SnRK2.2 and phosphorylation of CtAHA2 was observed by autoradiography. MBP was not phosphorylated. The mutated versions of the C-terminal fragment (S899P, S904L, or S931F) were phosphorylated as efficiently as the wild type. The dashed line in the Coomassie staining panel indicates the migration of the immunoprecipitated 3HA–SnRK2.2, which was estimated by overlapping with the anti-HA western blot. (B) His–OST1/SnRK2.6, ΔCABF2, MBP, and MBP–CtAHA2 were purified and added to the kinase assay as indicated. Autophosphorylation of His-OST1/SnRK2.6 and phosphorylation of ΔCABF2 was observed by autoradiography. Neither MBP nor MBP–CtAHA2 were phosphorylated. The experiments were repeated twice with similar results.