Abstract
Background
This study aimed to evaluate the role of biomarkers in the pathophysiological process induced by a Staphylococcus aureus strain obtained in a hospital environment. For this, we intraperitoneally inoculated groups of male BALB/c mice with S. aureus, using a clinical isolate (CI) of S. aureus.
Material/Methods
Mice were divided into groups according to time of euthanasia (24, 48, 72, 96, 120, 144, and 168 hours of infection). After being euthanized, blood samples were collected for quantification of microorganisms and leukocytes, as well as measurement of biomarkers of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), C-reactive protein (CRP), and Procalcitonin (PCT) by ELISA. Heart, kidneys, and lungs were removed for histopathological analysis, assessment of biomarkers of tissue expression by RT-PCR (polymerase chain reaction with reverse transcriptase), and quantification of microorganisms by real-time quantitative PCR (real-time PCR).
Results
The animals infected at between 120 hours and 168 hours had the highest blood levels of S. aureus. We observed that infection promoted increases in the levels of circulating neutrophils and monocytes. However, there was a reduction of circulating neutrophils and monocytes after 96 hours of infection. The infected mice also had increased levels of blood lymphocytes. In this model of infection with S. aureus, IL-6, CRP, and PCT demonstrated greater fidelity as markers of infection, since serum levels were elevated and lowered along with the number of circulating neutrophils and monocytes after resolution of the infection. The lungs showed hyperemia, with enlargement of the alveolar septa. On the other hand, infection with S. aureus did not promote visible change in histological tissue in the heart and kidneys.
Conclusions
In this model of infection with S. aureus, IL-6, CRP, and PCT demonstrated greater fidelity as markers of infection, since serum levels were elevated and lowered along with the number of circulating neutrophils and monocytes after resolution of the infection. We believe our results may provide a better understanding of the pathophysiology, as well as aid in the search for a more reliable method of diagnosis.
MeSH Keywords: Biological Markers, Sepsis, Staphylococcus aureus
Background
Sepsis is an acute inflammatory response against an infective organism, accompanied by a complex cascade of cellular and chemical interactions. The aim of the hyperdynamic sate is to eliminate the invasive organism from the body. Despite this protective mechanism, the acute response can lead to tissue damage and cause life-threatening complication if not properly and quickly treated [1]. The incidence of sepsis has increased in recent years due to the increase of the elderly population, longer hospitalization, and more invasive procedures; therefore, sepsis is growing in epidemiological importance [2]. Nosocomial infectious due to contaminated intravenous fluid, blood products, and medications have been documented [3]. The mortality rates of sepsis range from 12.8% for sepsis and 20.7% for severe sepsis, to up to 45.7% for septic shock [4].
The lipopolysaccharide (LPS) of gram-negative bacteria is known to be the main constituent inducing sepsis, which stimulates the release of endogenous mediators responsible for pathophysiological changes associated with high mortality [5]. Recent clinical studies have demonstrated that the causes of sepsis by gram-positive bacteria have been increasing, being responsible for 50% of cases of severe sepsis or septic shock in hospital intensive care units (ICUs) [6]. S. aureus is among these microorganisms, and is the leading cause of sepsis among gram-positive bacteria.
S. aureus was isolated from the blood of 52.4% of patients with sepsis, and the strains of S. aureus resistant to methicillin (MRSA) were recorded [7]. The peptidoglycan (PG) and lipoteichoic acids (LTA) of S. aureus, recognized by toll-like receptor 2 (TLR2) in monocytes/macrophages, induce inflammatory responses due to activation of nuclear factor kappa B (NFκB). This activation produces inflammatory mediators intended to eliminate the microorganism [8]. These mediators can be measured and used as markers of infection [9].
Biomarkers for sepsis include interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) and C-reactive protein (CRP), and procalcitonin (PCT) has recently received considerable attention. IL-6 is a cytokine acting in both innate immune responses, synthesized by monocytes, endothelial cells, fibroblasts, and other cells in response to microorganism stimulation [10]. TNF-α is the main mediator of the acute inflammatory response to gram-negative bacteria and other infectious microorganisms. This cytokine is responsible for many systemic complications in severe infections, such as disseminated intravascular coagulation [11].
CRP is an acute-phase protein produced by hepatocytes, and activates the complement system. It is used as a marker of inflammation and tissue damage [12]. Systemic levels of CRP are elevated in septic patients compared with non-septic patients [13]. PCT is a pro-hormone calcitonin, and studies show that their serum levels can be used to distinguish symptoms of sepsis from a non-infectious inflammatory response, being an important biomarker for differential diagnosis [14]. However, the sepsis diagnosis and assessment of severity becomes complicated due to the highly variable nature of its signs and symptoms, as well as by the lack of sensitive and specific laboratory tests in differentiating between infectious and noninfectious cases [15].
Therefore, there is a need for biomarkers that can be used to assess the severity and the evolution of infection. The aim of this study was to evaluate the role of biomarkers in sepsis induced by S. aureus. Our group established a model of infection in mice using a clinical isolate of S. aureus, and evaluated blood parameters, gene expression, and histopathology.
Material and Methods
Bacteria
A clinical isolate of S. aureus (CI) was used in this study. This isolate was obtained from a previous study that examined the presence of this organism in a hospital environment. In this study, clinical samples were obtained from ICU environments and equipment surfaces with a high possibility of contamination. In the same study, selection tests and isolation of sensitivity to antibiotics, of biofilm formation, and extraction of DNA for amplification of the mecA gene were done, which were positive for methicillin resistance. Pathogenicity tests for the detection of virulence genes also were done, which proved positive for staphylococcal enterotoxin type A genes (SEA), staphylococcal enterotoxin type B (SEB), leukocidin Panton-Valentine (PVL), and the IgG binding region and X region of protein A (Spa) [16]. Staphylococci were grown, cloned, and stored at −70°C. BHI (brain heart infusion) and mannitol salt agar (MSA) were used to cultivate the strain S. aureus for subsequent infection of the animals.
Animals
Forty-five male BALB/c mice (6–8 weeks old), provided by the University of Campinas, São Paulo, were used. BALB/c mice are widely used for the study of sepsis. BALB/c mice tend to generate humoral immunity and Th2 cytokines, a process that has sometimes been associated with development of sepsis [17]. The animals received food and water ad libitum. The study was conducted at the Laboratory of Microbiology and Immunology, Institute for Multidisciplinary Health, Federal University of Bahia, Brazil. The study was approved by the Ethics Committee on Animal Use (CEUA), University of São Paulo.
Experimental infection of mice
Groups of BALB/c mice were inoculated intraperitoneally with S. aureus. The strain was subcultured in mannitol salt agar at 37°C overnight. On the day of the experiment, a bacterial suspension was prepared by sodium chloride 0.9% solution and the inoculum (1 ml) was adjusted by spectrophotometer (1×108 CFU/mL). The animals were divided into groups according to the time of euthanasia (24, 48, 72, 96, 120, 144, and 168 hours of infection). The control groups of mice were inoculated with sterile saline and sacrificed at a specific time (168 hours). After onset of infection, the animals were sacrificed by decapitation.
Fluid and tissue collection
After euthanasia, blood samples were collected into tubes with EDTA and without EDTA. The samples with EDTA were used for total and differential blood count. The samples without EDTA were centrifuged at 2200 g for 5 minutes at 4°C. The serum was stored at −80°C for the measurement of biomarkers by ELISA (enzyme-linked immunosorbent assay). The coagulum was used for quantification of bacterial load by real-time PCR (qPCR).
Heart, kidneys, and lungs were removed and fractionated for analysis. A portion of the material was used for histopathology. The other part was stored at −80ºC for the performance of molecular techniques (RT-PCR and qPCR).
Total and differential blood cell count
For total blood cell count, 20 μl of blood (collected in EDTA) were mixed with 400 μl of liquid thinner and the sample was transferred to a Neubauer chamber to perform the leukocyte count in an increase of 40×. The differential blood cell count was stained by a Panoptic kit. One hundred leukocytes were counted using an immersion objective and the different types of leukocytes and their values were counted and recorded. Based on the total leukocyte count and percentage values found in the differential count, the absolute values were calculated for each leukocyte.
S. aureus quantification
The blood DNA was extracted according to the protocol of an Invitek (Stratec®) kit. DNA from tissue was extracted by the phenol-chloroform-thiocyanate using the Trizol® platform [18]. The real-time PCR was performed in duplicate, using the Applied Biosystems platform. This technique was performed by using TaqMan Probe using a basic amplification protocol. This protocol included, in a final reaction volume of 25 μl, 12.5 μl of Master Mix (Applied Biosystems), 1.12 μl of primer LTnucF (AAATTACATAAAGAACCTGCGACA), 1.12 μl of primer LTnucR (GAATGTCATTGGTTGACCTTTGTA) (20 pmol), 0.75 μl probe (10 mM), 7.0 μl of water, and 2.5 μl of DNA. Quantification was performed using an absolute quantification technique, based on a predetermined standard curve ranging from 107 to 10 microorganisms/uL.
Measurement of biomarkers
Sandwich ELISA was performed on biomarkers IL-6 (eBIOSCIENCE), TNF-α (eBIOSCIENCE), PCR (Life Diagnostics), and PCT (RayBio®). An ELISA 96-well plate was sensitized with a specific capture antibody and incubated overnight at 4°C. The wells were washed to remove free antibodies. After washing, the detection antibody was added. The wells were washed again and added to the secondary antibody conjugated to streptavidin peroxidase enzyme. After further washing, a substrate solution of tetramethylbenzidine (TMB) was added to the wells and the color developed in proportion to the amount of biomarker. The stop solution was added to the following section, changing the color from blue to yellow, and the color intensity was measured in an ELISA reader at 450 nm.
RNA isolation and RT-PCR
Total RNAm was extracted by the phenol-chloroform-thiocyanate using Trizol®. Extracted RNAm was quantified by spectrophotometry (NanoDrop ND-1000, Witec Ag, Littau, Switzerland). Equal amounts of RNAm per tissue were subjected to reverse transcription (RT) by Random oligo dT-primers with reverse transcriptase (Invitrogen®). After cDNA synthesis, polymerase chain reaction (PCR) amplification of the cDNA was performed for TNF-α, IL-6, PCT, and CRP. The amplified cDNA was electrophoresed in 1% agarose gels containing ethidium bromide, and quantities were analyzed by densitometry using ImageJ software (the Research Service Branch of the National Institute of Health, Bethesda, MD, USA). The relative expression of each gene was normalized to the intensity of a housekeeping gene, β-actin. The expression level of each gene is reported as a ratio relative to the level of normalized expression in a control sample.
Morphological and histological analysis
Heart, kidney, and lung tissues were fixed in methacarn solution (60% methanol, 30% chloroform, and 10% glacial acetic acid) for approximately 24 hours, embedded in paraffin, and cut into 4-μm sections. The slides were stained with hematoxylin and eosin to evaluate the intensity of inflammation. The measurements were performed with the aid of a computerized interactive image analysis (Image Pro Plus 4.1 imaging software from Media Cybernetics, Silver Spring, MD, USA) and based on the scoring standard. A video camera attached to the microscope optical system transmitted from each microscopic field, which was converted into a digital image.
Statistical analysis
Statistical analysis was performed using GraphPad Prism, 5.0 (GraphPad Software, San Diego, CA, USA). The comparisons made in the experiments and were determined by individual variability or error variation (s2) by analysis of variance one-way ANOVA followed by the Newman-Keuls test of comparison. The results are expressed as mean plus or minus the standard error of the mean. Statistical differences were considered significant at p<0.05.
Results
During the study period, all animals survived. No complications were observed related to induction of sepsis or treatment technique.
S. aureus quantification
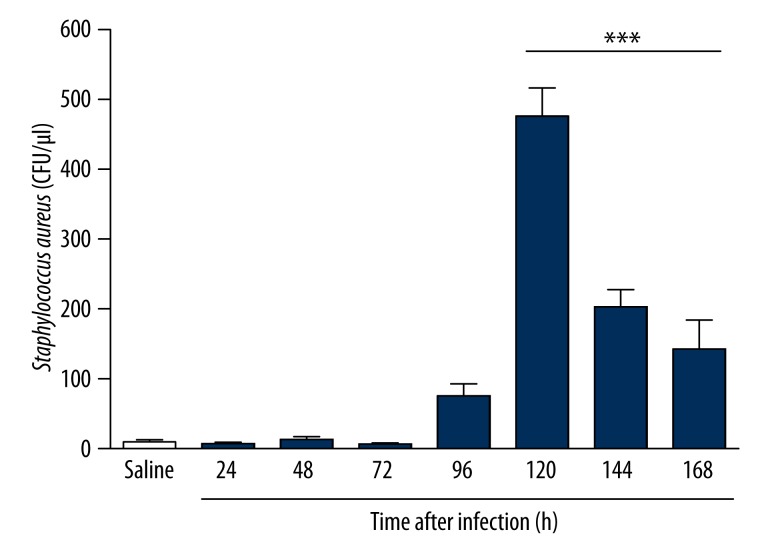
Infected animals showed higher levels of S. aureus in blood between 120 hours and 168 hours of infection (Figure 1). However, no S. aureus was detected by qPCR in any of the organs studied.
Figure 1.
Quantification in blood using qPCR of Staphylococcus aureus in a sepsis model induced by clinical strain in Balb/c mice infected for 7 days. Infected animals showed higher systemic levels of S. aureus within 120 hours and 168 hours of infection. *** p<0.05 vs. Saline.
Leukocyte count
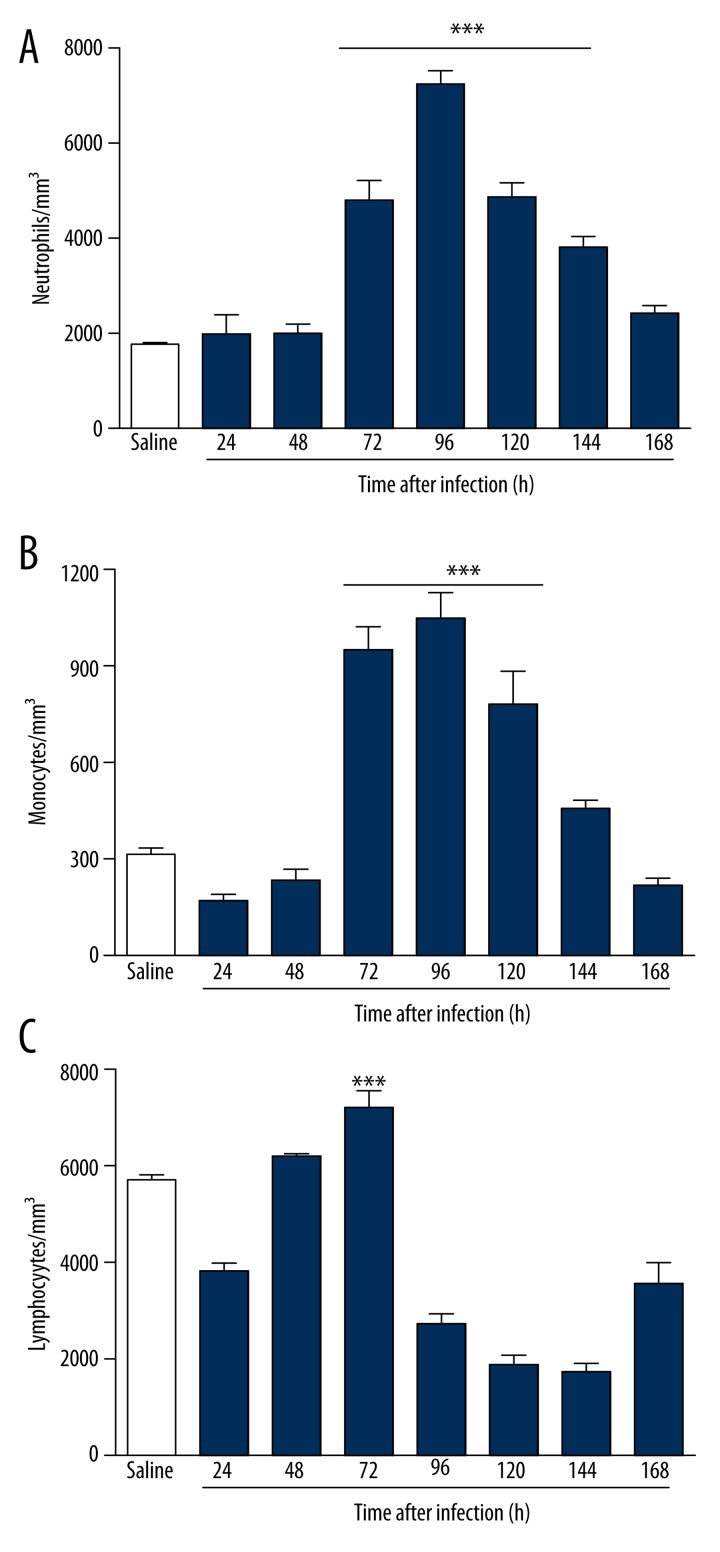
We observed that infection promoted increases in the levels of circulating neutrophils (Figure 2A) and monocytes (Figure 2B). However, there was a reduction of circulating neutrophils and monocytes after 96 hours of infection. The infected mice also had increased levels of blood lymphocytes (Figure 2C). The lymphocytes showed significant decreases after 72 hours of infection. However, there was a considerable tendency to return to baseline by 168 hours (Figure 2C).
Figure 2.
Blood leukocytes of BALB/c mice infected for 7 days with strain clinical isolate of S. aureus. The animals infected showed significant increases in blood levels of both neutrophils (A) and monocytes (B), peaking at between 96 and 120 hours. *** p<0.05 vs. Saline. The lymphocytes (C) showed considerable reduction after 72 hours of infection. *** p<0.05 vs. 96, 120, and 144 hours.
Biomarker serum levels and gene expression
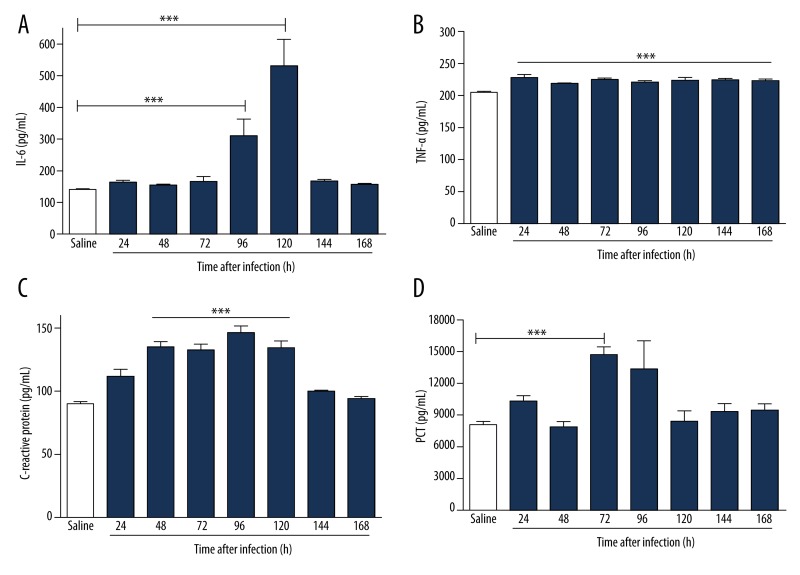
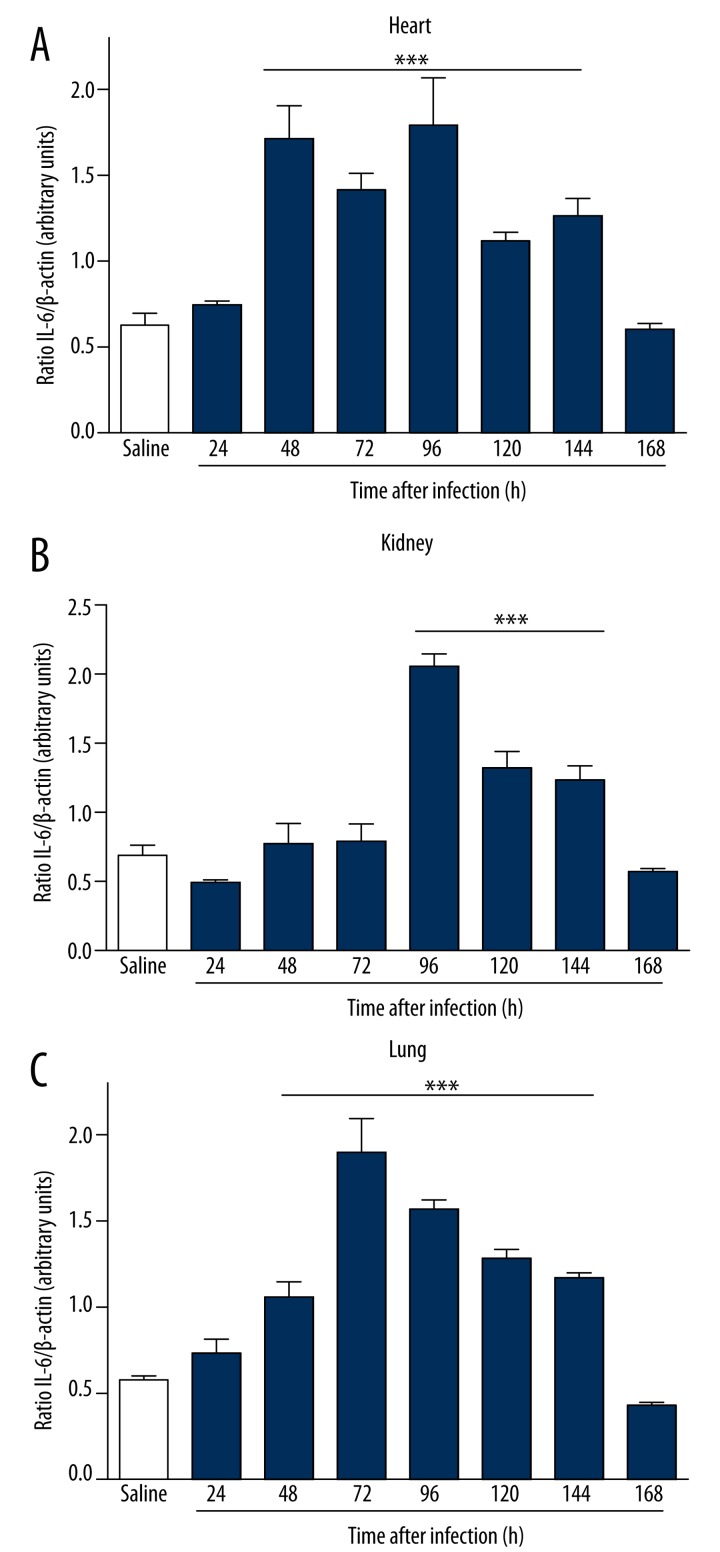
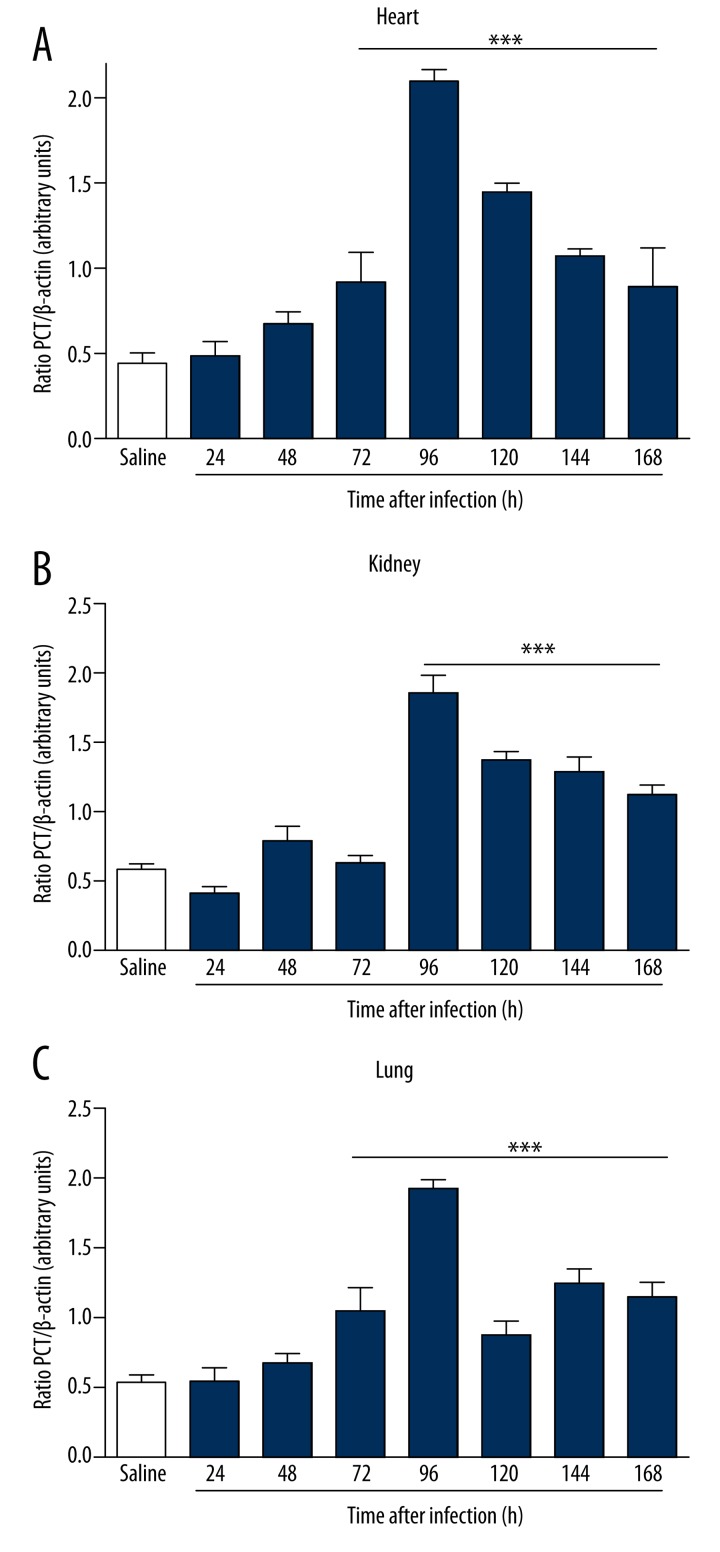
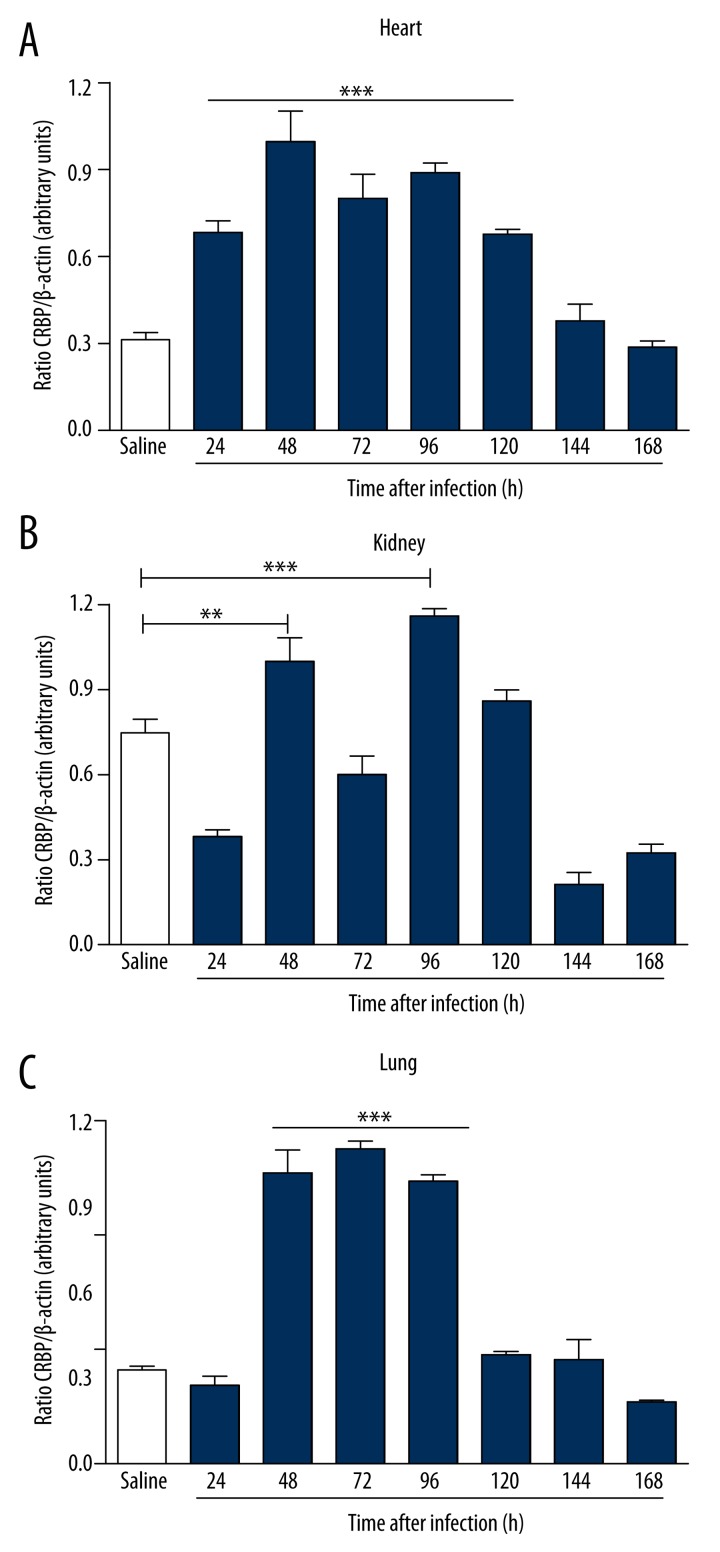
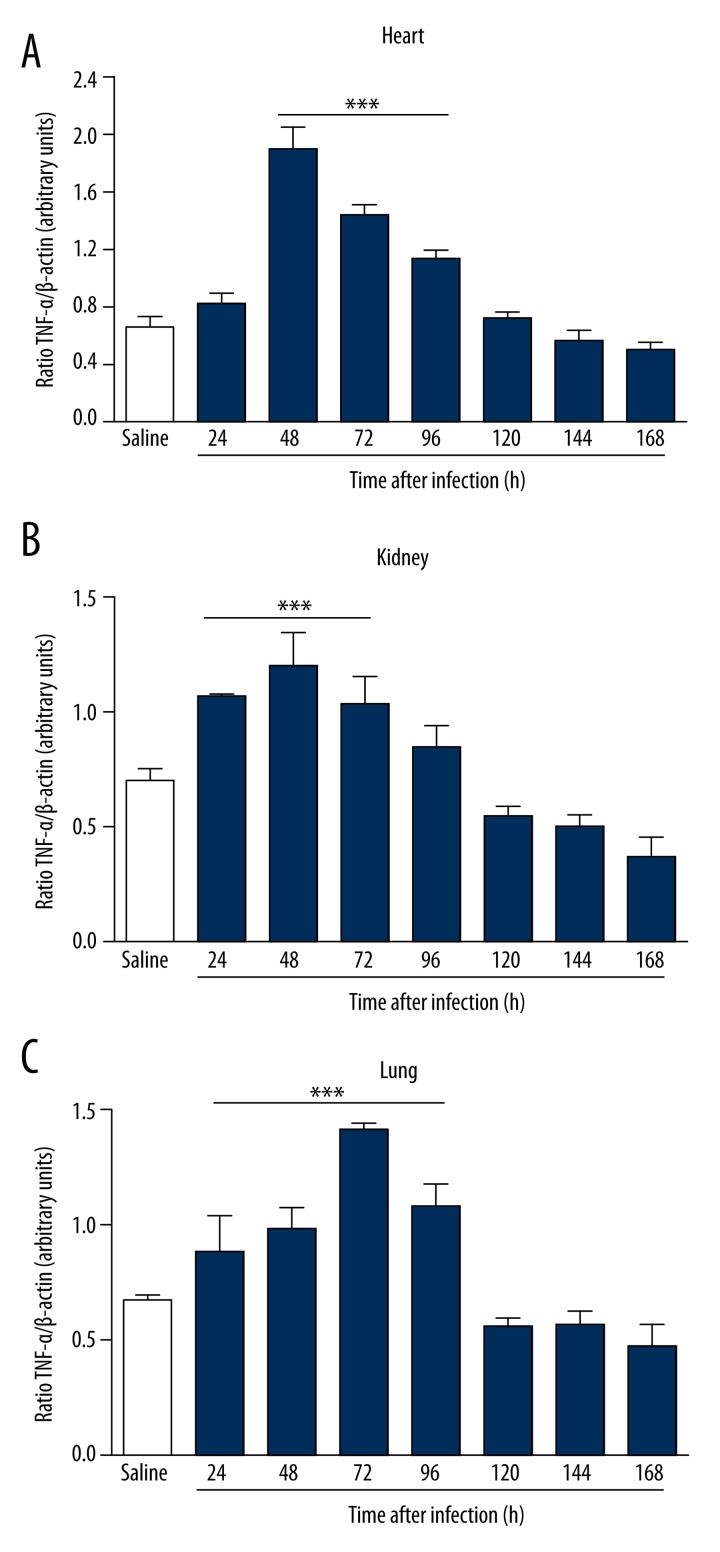
To study the mechanisms involved in the different response times of infection, we analyzed serum levels of TNF-α, IL-6, PCT, and CRP by ELISA after infection with S. aureus. In infected animals, the serum levels of biomarkers increased in direct relation to the time of infection. The increase in serum was more significant with respect to IL-6 (Figure 3A), CRP (Figure 3C), and PCT (Figure 3D) compared to the control group. A borderline significant correlation was observed in TNF-α dosages (Figure 3B). Infected animals showed an increased expression of IL-6 in the heart within 48 hours of infection (Figure 4A). In the kidney, the increase was more evident at 96 hours (Figure 4B), and in the lung there was a more pronounced increase of IL-6 at 72 hours of infection (Figure 4C). There was a significant increase in the expression of PCT, which was more evident within 96 hours of infection in the heart (Figure 5A), kidney (Figure 5B) and lung (Figure 5C). Infected animals also showed significant increases in gene expression of CRP in the heart within 24 hours of infection (Figure 6A), and 48 hours in the kidney (Figure 6B) and lung (Figure 6C), as well as an increased gene expression of TNF-α between 48 and 72 hours of infection in the heart (Figure 7A), kidney (Figure 7B), and lung (Figure 7C). In this model of infection with S. aureus, IL-6, CRP and PCT demonstrated greater fidelity as markers of infection, since serum levels were elevated and lowered along with the number of circulating neutrophils and monocytes after resolution of the infection.
Figure 3.
Plasma levels of biomarkers in BALB/c mice infected for 7 days with clinical isolate strain of S. aureus. The increase in plasma was more significant with respect to IL-6 (A) and PCT (D) compared to the control group. A borderline significant relation was observed in TNF-α (B) and CRP (C) dosages. *** p<0.05 vs. saline.
Figure 4.
Gene expression of IL-6 in BALB/c mice infected for 7 days with clinical isolate strain of S. aureus in heart (A), kidneys (B) and lungs (C). Infected animals showed an increased expression of IL-6 in the heart within 48 hours. In the kidneys the increase was more evident at 96 hours. In the lungs there was a more pronounced increase of IL-6 at 72 hours of infection. Electrophoresis represents the gene expression profile obtained from lung tissue. *** p<0.05 vs. saline.
Figure 5.
Gene expression of PCT in BALB/c mice infected for 7 days with clinical isolate strain of S. aureus in heart (A), kidneys (B), and lungs (C). There was a significant increase in the expression of PCT, more evident within 96 hours of infection in the heart, kidneys and lungs. Electrophoresis represents the gene expression profile obtained from lung tissue. *** p<0.05 vs. saline.
Figure 6.
Gene expression of CRP in BALB/c mice infected for 7 days with clinical isolate strain of S. aureus in heart (A), kidneys (B), and lungs (C). Infected animals showed significant increases in gene expression of CRP in the heart within 24 hours of infection, and 48 hours in the kidneys and lungs. Electrophoresis represents the gene expression profile obtained from lung tissue. *** p<0.05 vs. saline.
Figure 7.
Gene expression of TNF-α in BALB/c mice infected for 7 days with clinical isolate strain of S. aureus in heart (A), kidneys (B), and lungs (C). Infected animals showed an increased gene expression of TNF-α between 48 and 72 hours of infection in the heart, kidneys, and lungs. Electrophoresis represents the gene expression profile obtained from lung tissue. *** p<0.05 vs. saline.
Histological inflammation
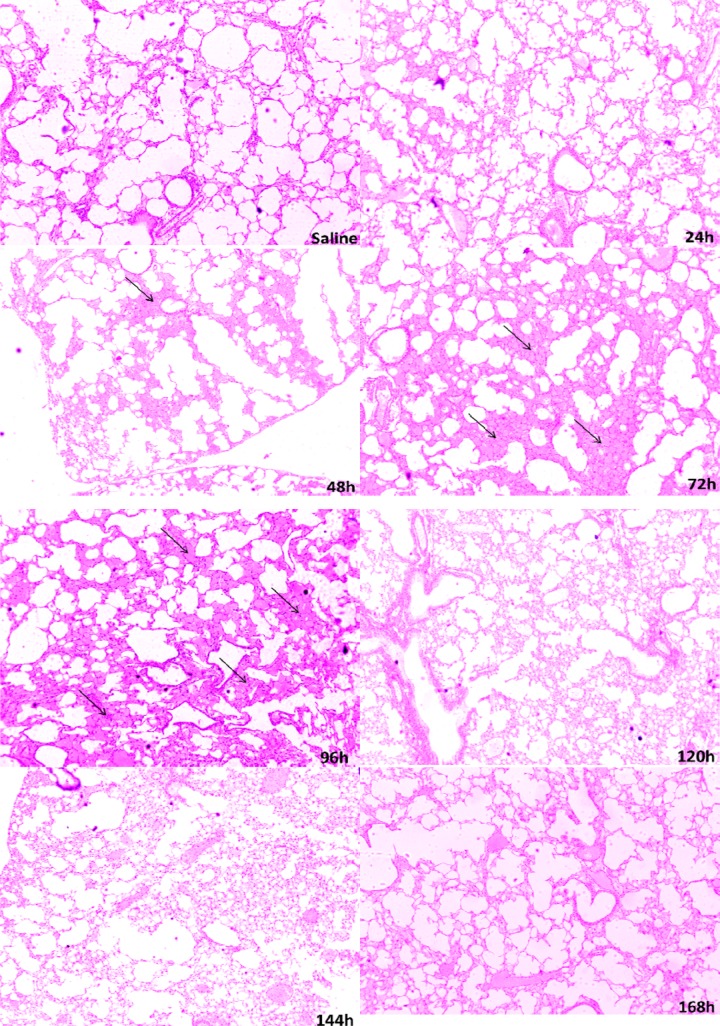
No histological changes were observed in infected animals in the heart or kidneys (data not shown). These animals differed in the histological analysis of the lungs. In the control group and 24 hours after infection, we observed clear alveoli without cellular infiltration. However, there was marked hyperemia with more pronounced enlargement of the alveolar septa between 48 and 96 hours, and it was less pronounced, but still present, within 144 hours and 168 hours (Figure 8).
Figure 8.
Representative photographs of lung tissue sections from male BALB/c mice infected with S. aureus. In the control group and 24 hours after infection, we observed clear alveoli without cellular infiltration. However, there was a marked hyperemia with more pronounced enlargement of the alveolar septa between 48 and 96 hours. It was less pronounced, but still present, at 144 hours and 168 hours. Arrows indicate enlarged alveolar septa.
Discussion
Infected animals showed higher levels of S. aureus in blood between 120 hours and 168 hours of infection. However, detection of S. aureus by qPCR was not observed in any of the organs studied. Similar findings were reported by Verdrengh and Tarkowski [19]. Bloodstream infections by S. aureus are among the most prevalent and difficult to treat and are associated with high mortality and high costs to the health care system [20]. According to Deitch (1990), the most probable mechanism of this increase in the bloodstream bacteria is injury of the gut mucosal barrier caused by an increase of microorganisms in the peritoneum, which results in villous edema and lesions of the lamina propria. Once the damaged mucosal barrier, microorganisms, and their components reach the lamina propria, they are phagocytized by resident macrophages and reach the mesenteric lymph nodes. From there they can spread to the thoracic duct, the veins of the portal system, liver, and lungs and initiate systemic circulation and then spread to other organs.
The ability to up-regulate virulence factors under stress stimuli (e.g., the host immune response) is a key factor in the persistence of S. aureus in the bloodstream and in the formation of secondary foci of infection [21]. This is because strains of S. aureus can adhere to and colonize the skin and mucous membranes of the nostrils to invade the bloodstream. Damage to the skin and the use of its virulence factors may result in bloodstream infections [22]. In the infection model of this study, peritonitis caused by the injection of large doses of S. aureus triggered a classic inflammatory response, but initially occurring where cytokines were released to combat invasion. These proinflammatory mediators assist local recruitment of neutrophils and monocytes to destroy the microorganism [23]. This may contribute to partial elimination of the microorganisms at the local site of infection, contributing to non-detection in the organs studied.
We also observed that infection with the ATCC strains and clinical isolates promoted increases in the levels of circulating neutrophils and monocytes and that infected mice had increased levels of blood lymphocytes. However, there was a reduction of circulating neutrophils and monocytes after 96 hours of infection. These findings are consistent with other studies previously reported in the literature [2,24,25]. Inappropriate activation of neutrophils contributes to the pathological manifestation of multiple organ failure [25]. Thus, the increase of neutrophils may have contributed to the elimination of the microorganism. In this study, we observed that there was a resolution of the infection. The increase and the decrease of S. aureus in the blood occurred simultaneously with neutrophils and monocytes at different times of infection, demonstrating that there was a beneficial effect on leukocyte function of eliminating the microorganism. This effect may be caused by cytokines such as the granulocyte colony-stimulating factor (G-CSF), which causes release of neutrophils from the bone marrow and promotes their maturation and activation [26]. Other experimental studies showed that there is a defect in leukocyte migration into tissues in humans with severe sepsis and septic shock, with consequent failure of immune system activity [27]. However, in this study we found there was a resolution of the infection and an observed increase and decrease of the simultaneous quantification of blood S. aureus, as well as neutrophils and monocytes at different times of infection, demonstrating that there was a beneficial effect on leukocyte function of eliminating the microorganism.
The lymphocytes showed significant decreases after 72 hours of infection. However, there was a considerable tendency to return to baseline by 168 hours. As shown here, as the infection resolves, the number of lymphocytes returns to baseline. During sepsis, lymphocyte depletion may occur [24]. These data are consistent with our findings, and show that apoptosis is essential for the resolution of inflammation, with a balance between the recruitment of leukocytes and their removal at the site of infection, which enhances host defenses so as to minimize cytotoxicity to the host. However, an important question about the death of lymphocytes during sepsis is whether it is beneficial or detrimental to the host. Although there is a possibility that lymphocyte death is beneficial because it causes down-regulation of the inflammatory response, this response can be detrimental because an abrupt loss of immune cells may compromise the ability of the host to eliminate infection [28].
In the present study, infection with S. aureus caused an increased release of IL-6. IL-6 participates mainly in fever induction and production of acute-phase proteins. Although the relevance of IL-6 effects in sepsis is not clear, this cytokine presents a better correlation with mortality in experimental models and in patients with sepsis [29]. The higher plasma levels of IL-6 increased the probability of death of the patient [30]. Studies with patients admitted to the emergency department with signs of infection reported a high plasma concentration of IL-6, which could predict bacteremia and death due to infection [31]. Neonates who developed sepsis showed high levels of IL-6 compared to uninfected babies [32].
PCT has been proposed as a marker of bacterial infection in critically ill patients [33]. Biju et al. [34] demonstrated the value of PCT as an early biomarker in radiation-induced bacteremia for mouse studies and suggested that clinical results from other septic conditions may apply to postradiation septicemia in humans. Unlike other biomarkers, it is not induced in autoimmune or neoplastic disorders, or in infections limited to a single organ, showing it to be extremely useful in the differential diagnosis of bacterial diseases [35]. According to Morgenthaler et al. [36], in a model of sepsis in primates, there was a large production of PCT in the liver, kidney, aorta, adipose tissue, ovaries, bladder, and adrenal glands. Chan, et al. [37] observed that infected patients showed increased plasma levels of PCT, showing it to be a good marker of severity of infection. According to Hausfater et al. [38], 195 patients with suspected infection showed elevated plasma levels of PCT. PCT appears to be a well-established biomarker of sepsis that fulfills several clinical needs: its responds both to infection and severity of inflammation and thus has a impact on therapy [39].
CRP is an acute-phase protein produced predominantly by hepatocytes and secreted into the serum. However, many lines of evidence indicate that it may be produced by other cells such as monocytes [40,41]. According to Dong and Wright [42], the CRP expression observed in the lungs is related especially to alveolar macrophages in mice subjected to infection. As a biomarker, Povoa [12] observed that patients with proven infection showed elevated plasma levels of CRP compared with those without proven infection. The plasma levels of CRP is determined exclusively by its rate of synthesis and disease activity [43]. Moreover, Kubler et al. [44] reported that patients treated with recombinant human activated proteín C showed lower relative risk of death than those who were not treated. However, it is not considered an exclusive marker for sepsis becauser it can be found high in other non-infectious conditions [37].
Studies show that the lungs are affected early in sepsis, and this can lead to serious injury in lung tissue [45]. Another study showed that septic lung infections were the primary source of infection in 47% of patients [46]. The lung injury caused after an abdominal infection is caused by contributions from neutrophils, platelets, the complement system, cytokines, and free radicals. The reactive oxygen species, released by neutrophils, contribute to increased lung injury [47]. Although S. aureus was not detected in the analyzed tissue, lung damage is also caused by bacterial components such as PG and LTA S. aureus. These components stimulate recruitment of neutrophils and macrophages by releasing cytokines and enzymes, causing injury to microcirculation [48].
The non-occurrence of histological changes visible in both heart and kidneys of infected animals may be due to the process of resolution of the inflammatory state. Although there was no visible damage to the tissues, we observed an inflammatory process by the release of inflammatory cells. The initial peritonitis caused by S. aureus triggered a response with release of cytokines, which may have aided in the recruitment of leukocytes in the first 24 hours. After resolution of the infection, both leukocytes and cytokines returned to baseline values, which may have contributed to the lack of visual changes in tissues.
Conclusion
The results of this study demonstrated that infection with S. aureus promoted a significant increase in blood levels of bacteria and local peritonitis caused by injury of the mucosal barrier, as well as increased blood levels of leukocytes because of the inflammatory process. This process contributed to partial elimination of the pathogen and its decrease in the blood. Visible change in histological lung tissue was observed, with neutrophil infiltration and enlargement of the alveolar septa, probably due to the release of inflammatory cytokines. However, infection with S. aureus did not lead to visible change in histological tissue in the heart and kidneys, and IL-6 and CRP proved to be more reliable as biomarkers. Plasma biomarker levels increased and decreased along with the levels of blood leukocytes and blood levels of bacteria, demonstrating that they are more sensitive markers of infection by S. aureus. This study proved important because there are no studies that relate this specific model of sepsis with its pathophysiological effects and participation of biomarkers. We believe our results may provide a better understanding of pathophysiology and aid in the search for a more reliable diagnostic approach
Acknowledgments
We thank Aricelma P. França for invaluable technical assistance and AcademicEnglishSolutions.com for revising the English.
Footnotes
Conflict of interest
All authors declare that there are no conflicts of interest or ethical issues.
Source of support: This study was supported by FAPESB (BOL0824/2011) and Programa de apoio a pesquisadores emergentes da UFBA (PRODOC 02/2011)
References
- 1.Gutierrez J, Hossam A, Lazarezcu R, et al. Effect of beta blockers on sepsis outcome. Med Sci Monit. 2009;15(10):CR499–503. [PubMed] [Google Scholar]
- 2.Cabrera-Perez J, Condotta SA, Badovinac VP, Griffith TS. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol. 2014 doi: 10.1189/jlb.5MR0114-067R. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achkar MA, Rogers JS, Muszynski MJ. Pantoea species sepsis associated with sickle cell crisis in a pregnant woman with a history of pica. Am J Case Rep. 2012;13:26–28. doi: 10.12659/AJCR.882588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas VJ, Lopez JC, Ruiz-Sanmartin A, et al. Severe sepsis mortality prediction with relevance vector machines. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2011; pp. 100–3. [DOI] [PubMed] [Google Scholar]
- 5.Grandel U, Fink L, Blum A, et al. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102(22):2758–64. doi: 10.1161/01.cir.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 6.Yi C, Cao Y, Mao SH, et al. Recombinant human growth hormone improves survival and protects against acute lung injury in murine Staphylococcus aureus sepsis. Inflamm Res. 2009;58(12):855–62. doi: 10.1007/s00011-009-0056-0. [DOI] [PubMed] [Google Scholar]
- 7.Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database – USA. Clin Infect Dis. 1999;29(2):259–63. doi: 10.1086/520195. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 9.Gullo A, Bianco N, Berlot G. Management of severe sepsis and septic shock: challenges and recommendations. Crit Care Clin. 2006;22(3):489–501. ix. doi: 10.1016/j.ccc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med. 2008;29(4):591–603. vii. doi: 10.1016/j.ccm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 12.Povoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002;28(3):235–43. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 13.Lausevic Z, Vukovic G, Stojimirovic B, et al. Kinetics of C-reactive protein, interleukin-6 and -10, and phospholipase A2-II in severely traumatized septic patients. Vojnosanit Pregl. 2010;67(11):893–97. doi: 10.2298/vsp1011893l. [DOI] [PubMed] [Google Scholar]
- 14.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25(4):609–34. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335(7625):879–83. doi: 10.1136/bmj.39346.495880.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos GB, Souza SG, Lob OT, et al. Isolation, molecular characteristics and disinfection of methicillin-resistant Staphylococcus aureus from ICU units in Brazil. New Microbiologica. 2012;35(2):183–90. [PubMed] [Google Scholar]
- 17.Ferguson NR, Galley HF, Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999;25(1):106–9. doi: 10.1007/s001340050795. [DOI] [PubMed] [Google Scholar]
- 18.Thomas LC, Gidding HF, Ginn AN, et al. Development of a real-time Staphylococcus aureus and MRSA (SAM-) PCR for routine blood culture. J Microbiol Meth. 2007;68(2):296–302. doi: 10.1016/j.mimet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus – induced arthritis and sepsis. Arthritis Rheum. 2000;43(10):2276–82. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Goetghebeur M, Landry PA, Han D, Vicente C. Methicillin-resistant Staphylococcus aureus: A public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007;18(1):27–34. doi: 10.1155/2007/253947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis. 2009;48(Suppl 4):S231–37. doi: 10.1086/598189. [DOI] [PubMed] [Google Scholar]
- 22.Weidenmaier C, Kokai-Kun JF, Kristian SA, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nature Med. 2004;10(3):243–45. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115(6):457–69. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 24.Azevedo LC. The many facets of sepsis pathophysiology and treatment. Shock. 2013;39(Suppl 1):1–2. doi: 10.1097/SHK.0b013e31828fad4a. [DOI] [PubMed] [Google Scholar]
- 25.Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 26.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–46. [PubMed] [Google Scholar]
- 27.Alves-Filho JC, de Freitas A, Spiller F, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis – a potential treatment of sepsis? Clin Infect Dis. 2005;41(Suppl 7):S465–69. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 29.Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence. 2014;5(1):154–60. doi: 10.4161/viru.27393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silman NJ. Rapid diagnosis of sepsis using biomarker signatures. Crit Care. 2013;17(6):1020. doi: 10.1186/cc13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moscovitz H, Shofer F, Mignott H, et al. Plasma cytokine determinations in emergency department patients as a predictor of bacteremia and infectious disease severity. Crit Care Med. 1994;22(7):1102–7. doi: 10.1097/00003246-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Chiesa C, Pellegrini G, Panero A, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem. 2003;49(1):60–68. doi: 10.1373/49.1.60. [DOI] [PubMed] [Google Scholar]
- 33.Mehanic S, Baljic R. The importance of serum procalcitonin in diagnosis and treatment of serious bacterial infections and sepsis. Mater Sociomed. 2013;25(4):277–81. doi: 10.5455/msm.2013.25.277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biju PG, Garg S, Wang WZ, et al. Procalcitonin as a Predictive Biomarker for Total Body Irradiation-Induced Bacterial Load and Lethality in Mice. Shock. 2012;38(2):170–76. doi: 10.1097/SHK.0b013e31825b2db3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotoula A, Gardikis S, Tsalkidis A, et al. Comparative efficacies of procalcitonin and conventional inflammatory markers for prediction of renal parenchymal inflammation in pediatric first urinary tract infection. Urology. 2009;73(4):782–86. doi: 10.1016/j.urology.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Morgenthaler NG, Struck J, Chancerelle Y, et al. Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. Horm Metab Res. 2003;35(5):290–95. doi: 10.1055/s-2003-41304. [DOI] [PubMed] [Google Scholar]
- 37.Chan YL, Tseng CP, Tsay PK, et al. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Crit Care. 2004;8(1):R12–20. doi: 10.1186/cc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausfater P, Garric S, Ayed SB, et al. Usefulness of procalcitonin as a marker of systemic infection in emergency department patients: a prospective study. Clin Infect Dis. 2002;34(7):895–901. doi: 10.1086/339198. [DOI] [PubMed] [Google Scholar]
- 39.Gentili A, Iannella E, Giuntoli L, Baroncini S. System for predicting outcome and for clinical evaluation in sepsis and septic shock: could scores and biochemical markers be of greater help in the future? Med Sci Monit. 2006;12(6):LE11–12. [PubMed] [Google Scholar]
- 40.Nelson GE, Mave V, Gupta A. Biomarkers for sepsis: a review with special attention to India. BioMed Res Int. 2015;2114:264–351. doi: 10.1155/2014/264351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156(12):4815–20. [PubMed] [Google Scholar]
- 43.Jincharadze N, Abelashvili D, McHedlishvili M, Kacharava M. Diagnostic value of C-reactive protein test at early-onset sepsis in preterm infants. Georgian Med News. 2006;130:87–91. [PubMed] [Google Scholar]
- 44.Kubler A, Mayzner-Zawadzka E, Durek G, et al. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Med Sci Monit. 2006;12(3):CR107–12. [PubMed] [Google Scholar]
- 45.Ozturk E, Demirbilek S, Begec Z, et al. Does leflunomide attenuate the sepsis-induced acute lung injury? Pediatr Surg Int. 2008;24(8):899–905. doi: 10.1007/s00383-008-2184-y. [DOI] [PubMed] [Google Scholar]
- 46.Martin G, Brunkhorst FM, Janes JM, et al. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13(3):R103. doi: 10.1186/cc7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Runcie C, Ramsay G. Intraabdominal infection: pulmonary failure. World J Surg. 1990;14(2):196–203. doi: 10.1007/BF01664873. [DOI] [PubMed] [Google Scholar]
- 48.Oberholzer A, Oberholzer C, Minter RM, Moldawer LL. Considering immunomodulatory therapies in the septic patient: should apoptosis be a potential therapeutic target? Immunol Lett. 2001;75(3):221–24. doi: 10.1016/s0165-2478(00)00307-2. [DOI] [PubMed] [Google Scholar]