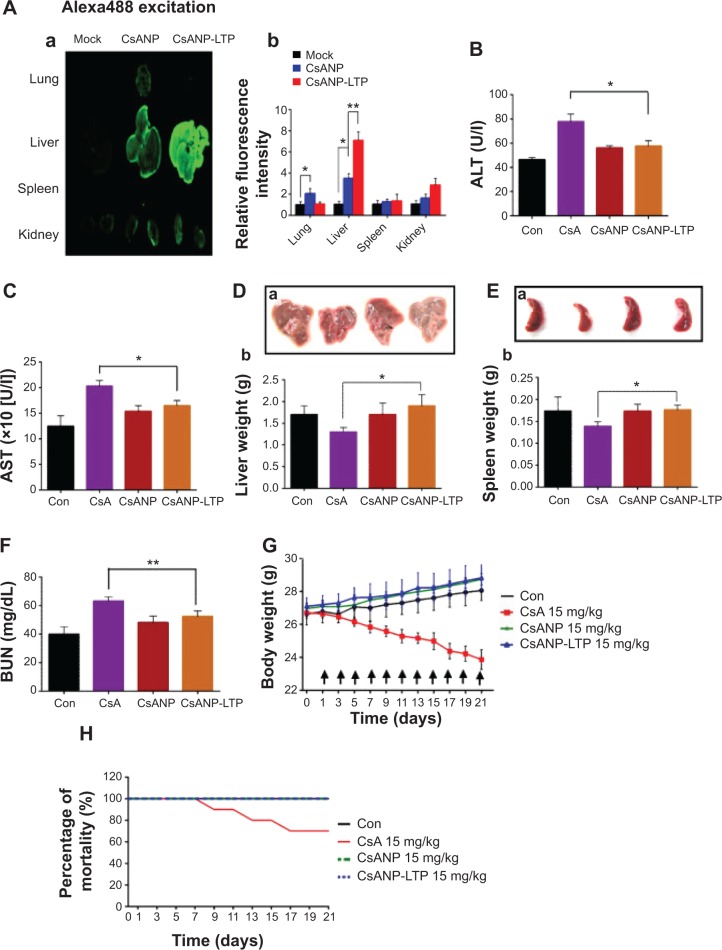
Figure 4.
Biodistribution and in vivo toxicity profile of CsANP-LTP in BALB/c mouse.
Notes: (A) Localization of Alexa-488-labeled nontargeted and targeted CsA poly (lactic-co-glycolic) acid nanoparticles (a) and relative fluorescence intensity (b) in lung, liver, spleen, and kidney of mice 24 hours after intravenous injection. The data are shown as the mean ± SD. *P<0.05, **P<0.01. A total of 24 male Balb/c mice were divided into four groups (n=6): Con (untreated) and CsA-, CsANP-, or CsANP-LTP-treated groups. Each treated group, received 15 mg/kg of CsA or equivalently loaded nanoparticle formulation intravenously at 48-hour time intervals for a period of 21 days. (B and C) Serum levels of ALT and AST. The data are shown as the mean ± SD. *P<0.05 versus CsA treatment. (D) Actual size of liver and kidney (a) and liver weight change (b). The data are shown as the mean ± SD. (E) Actual size of liver and kidney (a) and spleen weight change (b). The data are shown as the mean ± SD. *P<0.05. (F) Serum BUN levels. The data are shown as the mean ± SD. **P<0.01 versus CsA treatment. (G) Body weight. The data are shown as the mean ± SD. Arrows indicate time point of intravenous injection. (H) Toxicity-induced mortality rate.
Abbreviations: ALT, alanine aminotransferase; AST, alanine aminotransferase; BUN, blood urea nitrogen; Con, control; CsA, cyclosporine A; CsANP, cyclosporine A nanoparticle; CsANP-LTP, CsANP conjugated with liver-targeting peptide; SD, standard deviation; U/l, Units per liter.