Figure 5.
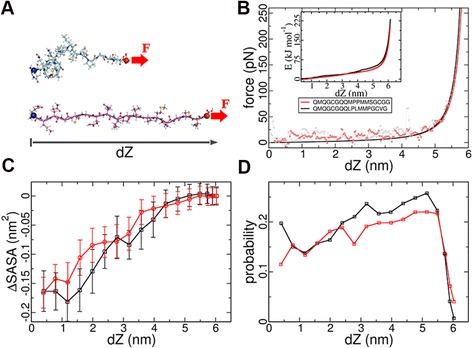
Molecular elasticity of Cnidoin peptides from MD simulations. (A) two representative (collapsed and extended) conformations of a Cnidoin peptide unit. To obtain force-extension curves, N-terminal C-alpha atoms (blue spheres) were fixed, while the C-termini (red spheres) were subjected to a force acted along the extension (red arrows). (B) mean forces calculated from umbrella sampling. The force profiles of two different Cnidoin repeat units (squares) were fitted with the worm-like chain model (solid lines). Resulting free energy profiles along the end-to-end distance are shown in the inset. (C) residue-averaged hydrophobic surface burial of two Cnidoin peptides measured by disappearance of solvent accessible surface area (ΔSASA). (D) PPII conformation content in the Cnidoin peptides along peptide extension. (C) and (D) use the same colour code as (B). MD, molecular dynamics; PPII, polyproline II.
