Table 1.
The PDE10A inhibition activity (IC50 nM) of cinnoline analogues
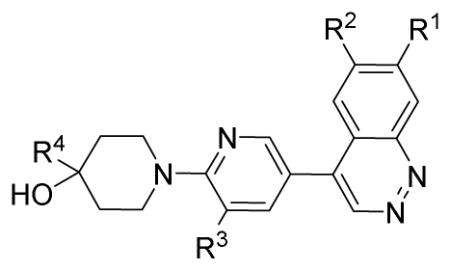
| |||||
|---|---|---|---|---|---|
| Compound# | R3 | R2 | R3 | R4 | IC50 (nM)a |
| 13a | -OCH3 | -OCH3 | -H | CH3 | 18.4 ± 6.0 |
| 13b | -OBn | -OCH3 | -H | CH3 | 4,250 ± 520 |
| 13c | -OCH3 | -OCH3 | -H | pyridin-3-yl | 14.0 ± 2.0 |
| 13d | -OBn | -OCH3 | -H | pyridin-3-yl | 1,550 ± 260 |
| 14a | -OH | -OCH3 | -H | CH3 | 10,800 ± 1,500 |
| 14b | -OH | -OCH3 | -H | pyridine-3-yl | 6,620 ± 600 |
| 26a | -OCH3 | -OCH3 | -CH3 | pyridine-3-yl | 1.52 ± 0.18 |
| 26b | -OCH3 | -OCH3 | -CH3 | 2-fluoro-pyridin-5-yl | 2.86 ± 0.10 |
| 26c | -O-CH2-O- | -CH3 | 2-fluoro-pyridin-5-yl | NAb | |
| 26d | -H | -H | -CH3 | 2-fluoro-pyridin-5-yl | NAb |
| 26e | -OCH3 | -OCH3 | -CH3 | 2-chloro-pyridin-5-yl | 9.8 ± 1.3 |
| 26f | -OBn | -OCH3 | -CH3 | 2-fluoro-pyridin-5-yl | 350 ± 23 |
| 26g | -OCH3 | -OCH3 | -Cl | pyridin-3-yl | 5.52 ± 0.33 |
| 26h | 2-(oxymethyl)-quinolinyl | -OCH3 | -CH3 | 2-fluoro-pyridin-5-yl | 518 ± 86 |
| 26i | fluoropropoxy | -OCH3 | -CH3 | 2-fluoro-pyridin-5-yl | 79.3 ± 3.6 |
IC50 value (mean ± SD nM) were determined in at least three experiments;
NA = no inhibition activity
